Showing Spotlights 73 - 80 of 137 in category All (newest first):
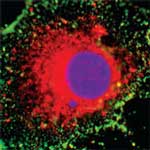 One of the major concerns regarding the potential risks of nanotechnology applications are possible toxic effects of nanoparticles. The concern is that these materials have the capacity to penetrate cells and potentially translocate to other cells, tissues and organs remote from the portal of entry to the body. This is considered to be a necessary step in the movement of particles deposited in the lung, entering the blood, acting upon cells in other tissues, manifesting ultimately in a physiological response. The importance of translocation in nanoparticle toxicology has been the subject of a recently completed nanotoxicology research project called 'Cell Pen', conducted by the Institute of Occupational Medicine in the UK together with a team of multi-disciplinary experts. As with so many previous nanotechnology risk review reports, it appears that this documents highlight more the uncertainties and the unknown than what is actually known about the interaction of nanoparticles with cells.
One of the major concerns regarding the potential risks of nanotechnology applications are possible toxic effects of nanoparticles. The concern is that these materials have the capacity to penetrate cells and potentially translocate to other cells, tissues and organs remote from the portal of entry to the body. This is considered to be a necessary step in the movement of particles deposited in the lung, entering the blood, acting upon cells in other tissues, manifesting ultimately in a physiological response. The importance of translocation in nanoparticle toxicology has been the subject of a recently completed nanotoxicology research project called 'Cell Pen', conducted by the Institute of Occupational Medicine in the UK together with a team of multi-disciplinary experts. As with so many previous nanotechnology risk review reports, it appears that this documents highlight more the uncertainties and the unknown than what is actually known about the interaction of nanoparticles with cells.
Jan 22nd, 2009
 In case you want to get up to date on what's happening around the world with regard to the development of risk governance for nanotechnology applications in food and cosmetics, a new report just out from the International Risk Governance Council (IRGC) provides a good overview. An early version of this report was originally written as a briefing paper for an expert workshop organized by the IRGC in 2008. It is also a companion to the IRGC Policy Brief due for publication in early 2009. While this report does not include any primary research, is is a useful primer for anyone who wants to get an overview of what is happening in this area. IRGC is an independent organisation whose purpose is to help the understanding and management of global risks that impact on human health and safety, the environment, the economy and society at large. The organization's focus on risk governance strategies for nanotechnology applications in food and cosmetics is based on rising public concerns.
In case you want to get up to date on what's happening around the world with regard to the development of risk governance for nanotechnology applications in food and cosmetics, a new report just out from the International Risk Governance Council (IRGC) provides a good overview. An early version of this report was originally written as a briefing paper for an expert workshop organized by the IRGC in 2008. It is also a companion to the IRGC Policy Brief due for publication in early 2009. While this report does not include any primary research, is is a useful primer for anyone who wants to get an overview of what is happening in this area. IRGC is an independent organisation whose purpose is to help the understanding and management of global risks that impact on human health and safety, the environment, the economy and society at large. The organization's focus on risk governance strategies for nanotechnology applications in food and cosmetics is based on rising public concerns.
Jan 20th, 2009
 Stakeholders attending the second annual "Safety for Success" dialogue last October in Brussels agreed that while many activities had taken place during the past year towards the responsible development and regulation of nanotechnologies more effort was needed. Discussion highlighted three areas that require coordinated effort from all parties: 1) Developing trustworthy information on products containing nanomaterials that are on or near the market, and on how they are tested. 2) Meaningful public engagement on the basis of shared definitions of nanotechnology. 3) Ongoing regulatory reviews to provide clear guidance to industry on how to interpret regulatory frameworks, and clear indications to the public about action being taken in cases where relevant risk data is limited or uncertain. In addition, the meeting identified a number of key points that need to be addressed in order to meet these three priorities.
Stakeholders attending the second annual "Safety for Success" dialogue last October in Brussels agreed that while many activities had taken place during the past year towards the responsible development and regulation of nanotechnologies more effort was needed. Discussion highlighted three areas that require coordinated effort from all parties: 1) Developing trustworthy information on products containing nanomaterials that are on or near the market, and on how they are tested. 2) Meaningful public engagement on the basis of shared definitions of nanotechnology. 3) Ongoing regulatory reviews to provide clear guidance to industry on how to interpret regulatory frameworks, and clear indications to the public about action being taken in cases where relevant risk data is limited or uncertain. In addition, the meeting identified a number of key points that need to be addressed in order to meet these three priorities.
Jan 9th, 2009
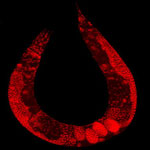 The flurry of recent announcements regarding reports, international cooperations, and new research activities that deal with the potential risks of manufactured nanomaterials is a clear indication that the field of nanotoxicology is gaining momentum - and not too soon. While there still is no coherent international approach to determining if and what risks are posed by what kind of nanotechnology materials, individual research groups are picking certain areas of concern and forge ahead with - often highly specific - toxicology studies. A lack of standards and definitions makes these early investigations hard to compare and sometimes they even contradict each other, a situation that is especially confusing in risk assessments of carbon nanotubes. Some studies, though, present findings that, on the face of it, are especially worrying in their potential implications and deserve much more attention to be sorted out one way or another. A recent report on the toxicity of metal nanoparticles in soil is such an example.
The flurry of recent announcements regarding reports, international cooperations, and new research activities that deal with the potential risks of manufactured nanomaterials is a clear indication that the field of nanotoxicology is gaining momentum - and not too soon. While there still is no coherent international approach to determining if and what risks are posed by what kind of nanotechnology materials, individual research groups are picking certain areas of concern and forge ahead with - often highly specific - toxicology studies. A lack of standards and definitions makes these early investigations hard to compare and sometimes they even contradict each other, a situation that is especially confusing in risk assessments of carbon nanotubes. Some studies, though, present findings that, on the face of it, are especially worrying in their potential implications and deserve much more attention to be sorted out one way or another. A recent report on the toxicity of metal nanoparticles in soil is such an example.
Jan 5th, 2009
 A new report prepared for the German Federal Ministry of Education and Research outlines an institutional model that meets the safety and security demands of human health, the environment and society. The report draws on an analysis of national and international approaches to nanotechnology regulation.
One of the key findings is that in the case of developing nanotechnologies, the place of classical regulation has been taken by precautionary measures such as observatories, voluntary codes of conduct and stakeholder dialogues. The development of an institutional model is proposed, the Scanning Probe Agency (SPA), as both a necessary and appropriate collective learning process and a means of generating public trust. Its guiding question would be: 'Is nanotechnology in good hands?'
A new report prepared for the German Federal Ministry of Education and Research outlines an institutional model that meets the safety and security demands of human health, the environment and society. The report draws on an analysis of national and international approaches to nanotechnology regulation.
One of the key findings is that in the case of developing nanotechnologies, the place of classical regulation has been taken by precautionary measures such as observatories, voluntary codes of conduct and stakeholder dialogues. The development of an institutional model is proposed, the Scanning Probe Agency (SPA), as both a necessary and appropriate collective learning process and a means of generating public trust. Its guiding question would be: 'Is nanotechnology in good hands?'
Dec 12th, 2008
 The UK's Royal Commission on Environmental Pollution in a new report clearly states that it found no evidence of harm to health or the environment from nanomaterials but it believes that the pace at which such new nanomaterials are being developed and marketed is beyond the capacity of existing testing and regulatory arrangements to control the potential environmental impacts adequately. A major conclusion of the report is that nanomaterials are hugely variable in their nature. They are not a uniform class of materials, and attempts to regulate or legislate solely on the basis of their size or how they are made are misguided. It is the functionality of nanomaterials, i.e. what they do and how they behave, that matters and this should form the basis of governance and regulation. The report makes a number of recommendations on how to deal with ignorance and uncertainty in the area of nanomaterials, which could also be applied to other areas of fast-paced technological development.
The UK's Royal Commission on Environmental Pollution in a new report clearly states that it found no evidence of harm to health or the environment from nanomaterials but it believes that the pace at which such new nanomaterials are being developed and marketed is beyond the capacity of existing testing and regulatory arrangements to control the potential environmental impacts adequately. A major conclusion of the report is that nanomaterials are hugely variable in their nature. They are not a uniform class of materials, and attempts to regulate or legislate solely on the basis of their size or how they are made are misguided. It is the functionality of nanomaterials, i.e. what they do and how they behave, that matters and this should form the basis of governance and regulation. The report makes a number of recommendations on how to deal with ignorance and uncertainty in the area of nanomaterials, which could also be applied to other areas of fast-paced technological development.
Nov 24th, 2008
 Titanium dioxide nanoparticles have become a commercially significant nanomaterial and are being used in products around the world - in cosmetics and sunscreen lotions, paint formulations, coatings, self-cleaning additives, even in antibacterial applications. The increased use of nanomaterials such as titania goes hand in hand with a growing number of reports on the risks associated with these materials, which have arisen because insufficient information has been gathered about their reactivity and stability once they leave the laboratory. Unfortunately, pinpointing every conceivable situation that nanoparticles could interact in is an enormous multi-parameter problem and solving this by experimental testing alone is not feasible due to the huge numbers of combinatorial variations. This is where theoretical predictions can help, by rapidly and systematically sampling possibilities, and highlighting where experimentalists should focus their attention.
Titanium dioxide nanoparticles have become a commercially significant nanomaterial and are being used in products around the world - in cosmetics and sunscreen lotions, paint formulations, coatings, self-cleaning additives, even in antibacterial applications. The increased use of nanomaterials such as titania goes hand in hand with a growing number of reports on the risks associated with these materials, which have arisen because insufficient information has been gathered about their reactivity and stability once they leave the laboratory. Unfortunately, pinpointing every conceivable situation that nanoparticles could interact in is an enormous multi-parameter problem and solving this by experimental testing alone is not feasible due to the huge numbers of combinatorial variations. This is where theoretical predictions can help, by rapidly and systematically sampling possibilities, and highlighting where experimentalists should focus their attention.
Nov 19th, 2008
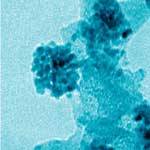 Adding yet another twist to the emerging debate about the potential risks of nanomaterials, researchers have demonstrated how difficult it is to map out the health effects of nanoparticles. They have shown that, even if a certain nanoparticle does not appear toxic by itself, the interaction between this nanoparticle and other common compounds in the human body may cause serious problems to cell functions. On one hand, this effect could be used to great advantage in nanomedicine for killing cancer cells. On the other hand, unfortunately, it is unknown at present whether the same effect could be observed with healthy cells as well. Since the number of possible combinations of nanoparticles and various biomolecules is immense, it is practically impossible to research them systematically. This latest example of the risk-benefit dichotomy of nanotechnology just shows how thin the line is between promising applications such as effective cancer killers and the unknown risks posed by unintentional effects of exactly the same applications.
Adding yet another twist to the emerging debate about the potential risks of nanomaterials, researchers have demonstrated how difficult it is to map out the health effects of nanoparticles. They have shown that, even if a certain nanoparticle does not appear toxic by itself, the interaction between this nanoparticle and other common compounds in the human body may cause serious problems to cell functions. On one hand, this effect could be used to great advantage in nanomedicine for killing cancer cells. On the other hand, unfortunately, it is unknown at present whether the same effect could be observed with healthy cells as well. Since the number of possible combinations of nanoparticles and various biomolecules is immense, it is practically impossible to research them systematically. This latest example of the risk-benefit dichotomy of nanotechnology just shows how thin the line is between promising applications such as effective cancer killers and the unknown risks posed by unintentional effects of exactly the same applications.
Nov 17th, 2008
 One of the major concerns regarding the potential risks of nanotechnology applications are possible toxic effects of nanoparticles. The concern is that these materials have the capacity to penetrate cells and potentially translocate to other cells, tissues and organs remote from the portal of entry to the body. This is considered to be a necessary step in the movement of particles deposited in the lung, entering the blood, acting upon cells in other tissues, manifesting ultimately in a physiological response. The importance of translocation in nanoparticle toxicology has been the subject of a recently completed nanotoxicology research project called 'Cell Pen', conducted by the Institute of Occupational Medicine in the UK together with a team of multi-disciplinary experts. As with so many previous nanotechnology risk review reports, it appears that this documents highlight more the uncertainties and the unknown than what is actually known about the interaction of nanoparticles with cells.
One of the major concerns regarding the potential risks of nanotechnology applications are possible toxic effects of nanoparticles. The concern is that these materials have the capacity to penetrate cells and potentially translocate to other cells, tissues and organs remote from the portal of entry to the body. This is considered to be a necessary step in the movement of particles deposited in the lung, entering the blood, acting upon cells in other tissues, manifesting ultimately in a physiological response. The importance of translocation in nanoparticle toxicology has been the subject of a recently completed nanotoxicology research project called 'Cell Pen', conducted by the Institute of Occupational Medicine in the UK together with a team of multi-disciplinary experts. As with so many previous nanotechnology risk review reports, it appears that this documents highlight more the uncertainties and the unknown than what is actually known about the interaction of nanoparticles with cells.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





