| Posted: Jun 04, 2014 |
A 'click' toward localized chemotherapy
|
|
(Nanowerk News) Platinum-based chemotherapy drugs are commonly used to treat a wide variety of solid tumors, including cancers affecting the breast, colon and lung. However, only a small amount of these anticancer drugs typically reaches the target organ system. This inefficiency not only reduces the efficacy of the drug; it also leads to severe side effects, ranging from nausea to kidney toxicity or deafness.
|
|
Charlotte Hauser and her co-workers at the A*STAR Institute of Bioengineering and Nanotechnology in Singapore have now developed a platform for the localized and sustained release of platinum-based anticancer therapeutics that overcomes many of these limitations. The research team’s novel gel formulation — a combination of specialized peptides and the drug oxaliplatin — led to dramatic growth inhibition when injected directly into the breast tumors of mice. In addition, the gel treatment produced a better tolerance profile than standard oxaliplatin drug therapy ("Ligation of anti-cancer drugs to self-assembling ultrashort peptides by click chemistry for localized therapy").
|
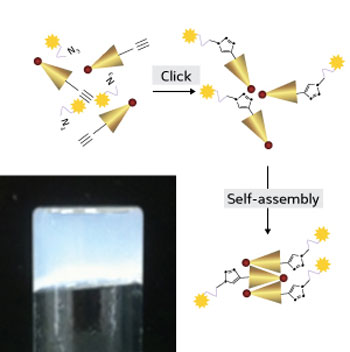 |
| The oxaliplatin-peptide conjugate, formed using click chemistry, self-assembles into a hydrogel (bottom left) that can be injected for localized drug delivery. (Image: RSC)
|
|
“Compared to the free drug, our injectable drug-loaded conjugate is just as effective in inhibiting tumor growth, but with lower systemic toxicity and higher localization in the target tissue,” says Charlotte Hauser.
|
|
Hauser’s team started with a unique class of ultrashort peptides that have the ability to spontaneously self-assemble and form hydrogels. The researchers attached oxaliplatin to the peptides using a technique known as ‘click’ chemistry, which enables the synthesis of complex molecules through the joining of multiple attached parts (see image). The resulting oxaliplatin-peptide hydrogels proved highly lethal against two human cell lines derived from cervical cancer and colon cancer tissues, respectively. Laboratory analyses showed that the hydrogels bound to the DNA of the cancer cells, arresting their replication cycle — just as free oxaliplatin does. Furthermore, the whole construct was biocompatible and generally non-immunogenic.
|
|
The researchers compared their hydrogel head-to-head with unmodified oxaliplatin in a breast cancer mouse model by injecting the drugs locally into the mouse tumors. Where the drug was delivered as a hydrogel, they documented higher rates of drug accumulation in the tumor — but lower levels of drug toxicity in the kidneys and livers — compared to the free oxaliplatin control. As a result, the mice given the hydrogel maintained more body weight, which can be used as a surrogate measure of their overall health.
|
|
“This combination product could serve as a tissue replacement device for the controlled release of important drugs that require localized and injectable treatments,” says Hauser. “We are exploring if this general approach can be utilized to attach a variety of other bioactive molecules.”
|

