| Posted: Aug 13, 2014 |
Pentagonal nanorods show catalytic promise
|
|
(Nanowerk News) Pentagonal nanorods have a unique morphology that confers interesting compositional and shape-dependent properties — including excellent stability and high catalytic activity — that make them excellent candidates for industrial catalysts. Now, researchers in Singapore have developed a simple chemical process to grow uniform pentagonal nanorods composed of gold and copper ("Multiply-twinned intermetallic AuCu pentagonal nanorods"). These new materials readily catalyze the direct alkylation of an amine with an alcohol, rendering them useful in the fields of materials chemistry and nanotechnology.
|
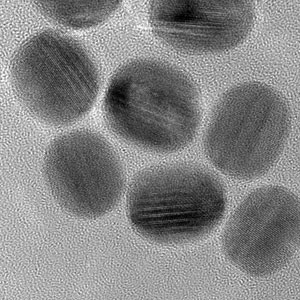 |
| A transmission electron microscopy image showing the uniformity of the gold–copper pentagonal nanorods. (© Royal Society of Chemistry)
|
|
“We successfully synthesized gold–copper pentagonal nanorods with controlled size and composition by a seed-mediated growth route,” explains lead researcher Jackie Ying from the A*STAR Institute of Bioengineering and Nanotechnology. The ‘seeds’ are multiple crystals of elongated gold decahedrons, joined together by shared faces — an arrangement known as multiply-twinning.
|
|
To create the nanorods, the team placed the gold seeds in a solution containing a copper precursor and applied heat — a process that produced nearly uniform pentagonal nanorods (see image).
|
|
Ying’s team showed that they could control the length of these nanorods by changing the amount of gold seeds added to the copper precursor. Adding a 1:1 ratio of gold to copper produced nanorods that grew approximately 15 nanometers in length while a 1:2 ratio produced nanorods approximately 19 nanometers long, and a 1:3 ratio produced nanorods approximately 24 nanometers long. The diameter of the nanorods remained the same, however, regardless of the ratio of metals used.
|
|
The ability to control the size and composition of the nanorods means it is easier to control the properties of the bimetallic gold–copper nanoparticles compared to nanoparticles made of just one metal, Yang explains.
|
|
Next, the team evaluated the catalytic activity of these gold–copper nanorods in a carbon–nitrogen-bond-forming reaction — the direct alkylation of an amine using an alcohol. “This hydrogen-borrowing strategy is an attractive synthetic method for the C–N bond formation as it is an environmentally friendly process which produces only water as a byproduct,” says Ying.
|
|
The nanorods were examined as catalysts for this reaction using the model substrates p-toluene sulphonamide and benzyl alcohol. “Our heterogeneous catalyst showed higher catalytic activity toward the C–N coupling reaction and better recyclability compared to commercially available catalysts,” Ying says.
|
|
Beyond catalysis, Ying predicts these new materials could be useful in electronics, chemical sensing and even biomedicine. Her team now plans to use the nanorods as seeds themselves to synthesize nanoparticles comprised of a gold–copper core surrounded by a shell of another material, such as platinum, for energy applications.
|

