| Posted: Sep 08, 2014 |
Stealthy and sticky: The chemical battle inside instantaneous graphene energy storage devices
|
|
(Nanowerk News) When you're merging onto the Beltway around the nation's capital, you want to go from 20 to 70 mph now. Supercapacitors, often built from a two-dimensional material called graphene, have the potential to provide electric or hybrid cars with the energy needed to safely merge into traffic, but different studies of the devices' performance give almost random performance data. The suspected culprits were tiny flaws on the graphene electrode. But, what, exactly, was happening?
|
|
A trio of scientists at Pacific Northwest National Laboratory (PNNL) found that two groups of atoms were slipping onto the surface and causing ions to stick, keeping charged particles from doing their job of storing and releasing electricity ("Effects of Oxygen-Containing Functional Groups on Supercapacitor Performance").
|
|
"We've just taken some of the guesswork out of supercapacitor electrode construction," said Dr. M. Vijayakumar, who led the study at the national laboratory.
|
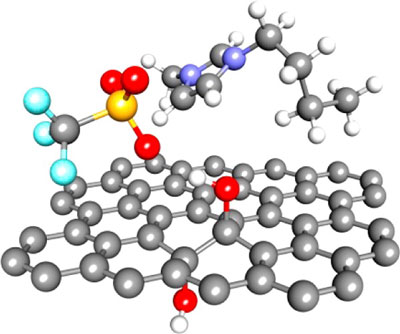 |
| Using molecular dynamics simulations, scientists discovered that on the surface of graphene (gray structure), hydroxyl groups (red and white) cause ions to stick, keeping charged particles from moving as needed to store and release electricity in a supercapacitor. (© American Chemical Society)
|
|
Why It Matters
|
|
Future cars could contain supercapacitors and batteries, but no gas tank. If you want to accelerate quickly, the supercapacitor will give your car a boost. The battery will provide the constant energy for regular driving. Braking will channel energy back into the supercapacitor, recharging it. Understanding what happens inside graphene-based supercapacitors, specifically the graphene electrodes' capacitance, or ability of ions to congregate on the surface, gives scientists insight to design devices that can improve electric cars' acceleration.
|
|
Methods
|
|
In the scientific literature, capacitance values for graphene electrodes were not consistent. The team wanted to know why the values differed. Funded by the Laboratory Directed Research and Development Office at PNNL, the team used molecular dynamics simulations to examine the electrolyte ions as they interacted with the graphene sheets. Based on that ion distribution, the team was able to determine the electrical charge or lack thereof on the electrodes.
|
|
"It was challenging to work out how to carry out the simulations and work out the general approach that can now be applied to other systems," said Dr. Sebastien Kerisit, a materials scientist at PNNL who led the simulations.
|
|
The team found that hydroxyl groups, formed from one oxygen and one hydrogen atom (-OH), change the electrode's capacitance. The hydroxyl groups form strong bonds with the ions in the electrolyte and prevent them from moving. "It's like putting Velcro hooks on the electrode," said Dr. Birgit Schwenzer, a materials scientist at PNNL who validated the simulation's performance against experimental data. "It makes the ions stick. They don't move like you need them to move."
|
|
Another common defect on the surface is epoxy groups, formed from one oxygen bridging two carbon atoms to form a three-membered ring. The epoxy groups did not help the electrode's performance but did not reduce it either.
|
|
The computational simulation approach devised by the team will allow the analysis of other defects on graphene. "We can isolate different defect types, which is hard to do experimentally even with all of the characterization tools available," said Vijayakumar.
|
|
What's Next?
|
|
The team is now working on building supercapacitors with specific defects to determine how the electrode and electrolyte behave. Their goal is to build a supercapacitor with one specific type of defects and an electrolyte that lets them control the defects to see if they can get the material performance needed for faster acceleration in electric cars.
|

