| Posted: May 20, 2015 |
What happens inside a membrane
|
|
(Nanowerk News) Little is known about how the proteins forming ion channels – the “pores” on the cell membrane - change when they open and close, especially the portion that is “embedded” in the membrane. Scientists at SISSA have invented a method, based on the combined and innovative use of known techniques, which allowed them to observe in detail a specific membrane protein and its structural changes. The study has just been published in Nature Communications ("Conformational rearrangements in the transmembrane domain of CNGA1 channels revealed by single-molecule force spectroscopy").
|
|
A new SISSA study has achieved two important results with a single effort: to devise an innovative method to analyse the structure of biological proteins immersed in their physiological context, and to closely observe a major ion channel, discovering as yet unseen details about its opening/closing mechanism.
|
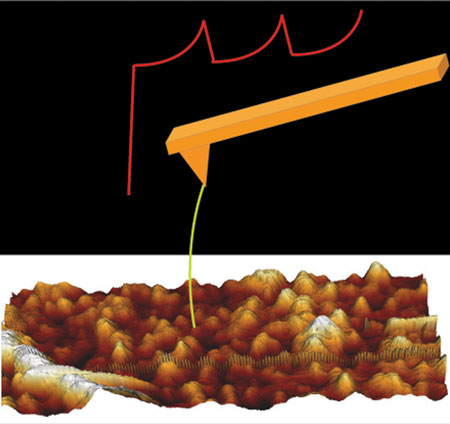 |
| Single-molecule force spectroscopy. (Image: SISSA)
|
|
“Strictly speaking, it’s not a new technique, but rather a novel combination of available techniques” explains Sourav Maity, researcher at the International School for Advanced Studies (SISSA) of Trieste. Maity has just published what is actually his SISSA PhD thesis in Nature Communications, an enviable and exceptional result at least at the start of one’s academic career.
|
|
“This method provides excellent spatial resolution for viewing molecular structure, with the advantage that we don’t have to purify the molecules, which is something we have to do with conventional spectroscopy, the technique currently offering the most precise images,” explains Vincent Torre, the SISSA professor who coordinated the study. “The method makes it possible to study molecules in their natural ‘environment’, in situ, which is impossible with the other technique”.
|
|
By using this method Maity and colleagues were able to observe things never seen before. The study investigated a protein of the cyclic nucleotide-gated (CNG) ion channel family which is found in the light-sensitive rod cells of the retina and is important in phototransduction (transformation of a light stimulus into a electrical nerve signal).
|
|
“We’re the first to have closely and exhaustively observed the CNG transmembrane domain, the portion immersed in the cell membrane lipid layer, and to have documented that the opening and closing of the channel corresponds to a structural change in the molecule”, comments Maity. This way, the closing and opening mechanism becomes less mysterious, and this is important as one day we hope to be able to use these “gateways”, for example, to deliver specific medications directly into the cell.
|
|
Maity and colleagues also discovered that the CNG channel protein “at rest”, i.e., in its stable conformation, is not always the same. “It presents in at least three forms, something that was completely ignored until now. This structural variability does not, however, correspond to different working mechanisms”, explains Maity. “It’s just three stable conformations that the protein can take on but that don’t affect its properties”.
|
|
More in detail …
|
|
The method used in the study is a combination of different techniques. It is based on single-molecule force spectroscopy, which exploits atomic force microscopy. In the study, this technique was used alongside electrophysiology and bioinformatics and the use of mutagenesis. This provides a detailed image of molecular structure without the disadvantage of having to purify the molecules (and remove them from their natural environment) as in x-ray spectroscopy.
|
|
“By using this technique, we can learn about a large number of proteins in greater detail than obtained so far and, more importantly, we can do it in situ, for example, without having to remove a membrane protein from the membrane itself” explains Maity, who is now continuing his research by analysing other proteins, such as rhodopsin, a pigment central to vision that is normally contained in different forms in retinal rod cells. Other researchers who took part in the study were Monica Mazzolini, Manuel Arcangeletti and Paolo Fabris, all SISSA research fellows.
|

