| Posted: Jul 09, 2015 |
Watching protein crystal growth in living cells in real time
|
|
(Nanowerk News) X-ray crystallography is a fundamental tool used for identifying the atomic and molecular structure of many materials which can form crystals, such as metals or minerals, as well as various inorganic, organic and biological molecules. For example, the three-dimensional structure of a protein determines its function; consequently, structural insights into proteins at atomic resolution are important to understand the machinery of life or to develop new specifically designed drugs for medical applications.
|
|
This technique requires sufficiently large crystals to obtain structural insights at atomic resolution, routinely obtained in vitro by time-consuming screening. However, in the past few years, some successful structural information was obtained from tiny protein microcrystals grown within living cells, offering exciting new possibilities for proteins that do not form crystals in vitro. Unfortunately, crystal formation within a living cell still represents a spontaneous event that is detected by chance.
|
|
In the present study, published at the journal Structural Dynamics ("Real-time investigation of dynamic protein crystallization in living cells"), the researchers observed crystal growth in real time, gathering important information about in vivo crystal formation. For that they used two different proteins: firefly luciferase and a truncated version of an avian reovirus protein, called muNS, fused to GFP, which allowed them to monitor by fluorescence the initial steps of the crystal formation in the cells.
|
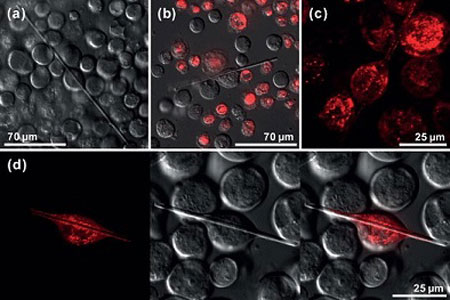 |
| Morphology and intracellular localization of in vivo firefly luciferase crystals.
|
|
The CiQUS researchers detected the spontaneous formation of protein crystals inside cells when expressing two different proteins with the baculovirus system. One of them (muNS-GFP) has a fused GFP moiety, what allows to observe in real time the initial events of the crystallization process.
|
|
The Molecular Virology Group, led at CiQUS by Prof. J. Benavente and Prof. Martínez Costas, studied the molecular biology of avian reovirus. Based on this work, they have developed and patented a protein-tagging technology platform, which they are currently adapting to other expression systems and studying its potential biotechnological applications.
|
|
This research has been coordinated by Prof. Lars L. Redecke (Center for Structural and Cell Biology in Medicine, University of Lübeck, Germany), in collaboration with the CiQUS (University of Santiago de Compostela, Spain), the Interfaculty Institute of Biochemistry (University of Tübingen, Germany) and the Institute of Biochemistry and Molecular Biology (University of Hamburg).
|

