| Posted: Aug 05, 2015 |
Sandcastles inspire new nanoparticle binding technique
(Nanowerk News) If you want to form very flexible chains of nanoparticles in liquid in order to build tiny robots with flexible joints or make magnetically self-healing gels, you need to revert to childhood and think about sandcastles.
|
|
In a paper published this week in Nature Materials ("Nanocapillarity-mediated magnetic assembly of nanoparticles into ultraflexible filaments and reconfigurable networks"), researchers from North Carolina State University and the University of North Carolina-Chapel Hill show that magnetic nanoparticles encased in oily liquid shells can bind together in water, much like sand particles mixed with the right amount of water can form sandcastles.
|
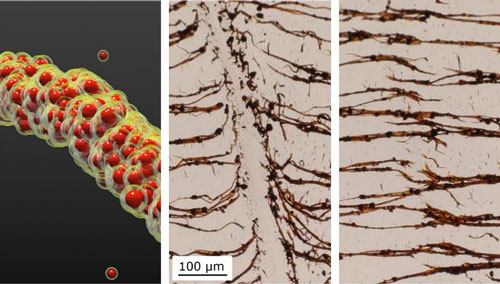 |
| NC State researchers develop a technique to assemble nanoparticles into filaments (left) in liquid. The filaments can be broken (middle) and then re-assembled (right). (Image courtesy of Bhuvnesh Bharti)
|
|
“Because oil and water don’t mix, the oil wets the particles and creates capillary bridges between them so that the particles stick together on contact,” said Orlin Velev, INVISTA Professor of Chemical and Biomolecular Engineering at NC State and the corresponding author of the paper.
|
|
“We then add a magnetic field to arrange the nanoparticle chains and provide directionality,” said Bhuvnesh Bharti, research assistant professor of chemical and biomolecular engineering at NC State and first author of the paper.
|
|
Chilling the oil is like drying the sandcastle. Reducing the temperature from 45 degrees Celsius to 15 degrees Celsius freezes the oil and makes the bridges fragile, leading to breaking and fragmentation of the nanoparticle chains. Yet the broken nanoparticles chains will re-form if the temperature is raised, the oil liquefies and an external magnetic field is applied to the particles.
|
|
“In other words, this material is temperature responsive, and these soft and flexible structures can be pulled apart and rearranged,” Velev said. “And there are no other chemicals necessary.”
|
|
“This research was the result of collaboration initiated by the NSF Materials Research Science and Engineering Center that facilitates interactions between Triangle universities.” said Michael Rubinstein, John P. Barker Distinguished Professor of Chemistry at UNC and one of the co-authors of the paper.
|

