| Posted: Oct 21, 2015 |
Nanoislands and skeletal skin boost fuel cell performance
(Nanowerk News) Selective electrodeposition of tin oxide on platinum alloy nanoparticles provides an effective and robust catalyst for polymer electrolyte fuel cells, report researchers in Japan ("Surface-Regulated Nano-SnO2/Pt3Co/C Cathode Catalysts for Polymer Electrolyte Fuel Cells Fabricated by a Selective Electrochemical Sn Deposition Method").
|
|
Polymer electrolyte fuel cells (PEFC) could provide an alternative to traditional fossil fuel power, but higher performance and durability under harsh conditions are needed before PEFC vehicles can be considered commercially viable. Now researchers at the University of Electro-Communications, the University of Tokushima and Japan Synchrotron Radiation Research Institute in Japan have synthesised catalysts from platinum cobalt (PtCo3) nanoparticles on carbon (C) with tin oxide (SnO2) nanoislands and shown that they perform better than any previously reported.
|
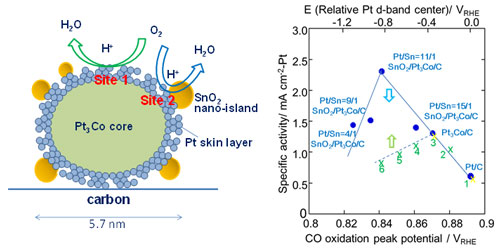 |
| Left: Model structure and proposed oxygen reduction reaction activity sites (sites 1 and 2) for the nano-SnO2-decorated Pt3Co/C with Pt skeleton surface with compressive strain and defects/dislocations. Right: Correlation between the specific activity (SA) and the CO-stripping peak potentials for the SnO2/Pt3Co/C with different platinum/tin ratios: Pt/Sn = 4/1, 9/1, 11/1, and 15/1), Pt3Co/C, and Pt/C. The green dotted line represents averages from data for catalysts reported in other work - (1) Pt/C, (2) Pt3Ni/C, (3) Pt3Co/C, (4) Pt3Fe/C, (5) Pt3V/C, and (6) Pt3Ti/C. These are also shown by scaling of the relative d-band center, where the values of Pt/C and Pt3Co/C (yellow ×’s over blue dots) from reports of data for the other catalysts were normalized to the values in the present study. (click on image to enlarge)
|
|
Fuel cell research has focused on platinum alloys and transition metal oxides to improve on the durability and catalytic performance of platinum on carbon. Previous work with SnO2 islands grown on platinum tin alloy with carbon had already shown some improvement in the oxygen reduction reactions that occur in fuel cells. However growing islands of only SnO2 on other alloys posed a challenge.
|
|
Now Yasuhiro Iwasawa at the University of Electro-Communications and his colleagues have grown SnO2 islands on Pt3Co nanoparticles on carbon (Pt3Co/C) by selective electrochemical deposition of tin metal, which is then oxidized. The addition of the SnO2 nanoislands doubled the catalytic performance of the Pt3Co/C catalysts. In addition they were undamaged after undergoing 5000 cycles of voltage changes to test their durability.
|
|
The structure the Pt3Co nanoparticles form has a Pt3Co core surrounded by a platinum skin that has a rough – “skeleton” – morphology. The researchers attribute the high catalytic performance in part to efficient electronic modification specifically at the platinum skin surface, and in particular to the unique property of the SnO2 nanoislands at the compressive platinum skeleton-skin surface.
|
|
“In general, adhesion of transition metal oxides on carbon induces depression of the electrical conductivity of the carbon,” explain the researchers in their report. “Hence, the selective nano-SnO2 decoration on the Pt-enriched-surface nanoparticles provides a significant advantage as a cathode catalyst.”
|
|
Background
|
|
Polymer electrolyte fuel cells
|
|
Polymer electrolyte fuel cells consist of two porous polymer membranes. On one side hydrogen gas molecules give up electrons and on the other oxygen gas molecules accept electrons completing a current circuit. The ions can then penetrate the membrane and combine to form water.
|
|
Polymer electrolyte fuel cells have several advantages over conventional fuel as they do not deplete the limited supplies of fossil fuels, and the waste products are water and heat, and therefore relatively non-polluting. The efficiency of fuel cells has already highlighted their potential for powering small vehicles.
|
|
Redox
|
|
The formation of hydrogen and oxygen ions from the gas molecules are referred to as redox reactions from the term ‘reduction’ and ‘oxidation’. In fuel cells neutral oxygen molecules are reduced to negatively charge oxygen ions with a charge of -2. The oxidation number is thus ‘reduced’ from 0 to -2. In contrast, ionisation of hydrogen molecules to positively charge hydrogen ions (that is single protons) increases the oxygen number by one – ‘oxidation’.
|
|
Catalysts are used to increase the efficiency of the redox reactions in fuel cells to improve the power and current density. The efficiency of the catalysts is measured in terms of the oxygen reduction reaction (ORR) activity.
|
|
Improving ORR
|
|
The researchers measured the potential difference required for other reactions in the presence of their catalyst to determine how the additional SnO2 islands improved the ORR. Their observations suggest that strain at the nanoislands on the Pt3Co nanoparticles modifies the electronic structure so that the centre of the electron d band is decreased. This decreases oxygen adsorption and improves the performance of the catalyst. In addition there is an increase in the proton affinity of the platinum near the nanoislands, which significantly enhances the ORR further still.
|

