| Posted: Mar 15, 2016 |
New research maps nanoparticles in tissue using hyperspectral imaging
(Nanowerk News) Sara Brenner, MD, MPH and colleagues at SUNY Polytechnic Institute (SUNY Poly), the George Washington School of Medicine and Health Sciences, and SUNY Stony Brook have demonstrated a novel method for the rapid visualization and identification of engineered nanoparticles in tissues. This research, published in Microscopy Research and Technique ("Hyperspectral imaging of nanoparticles in biological samples: Simultaneous visualization and elemental identification"), presents a method for utilizing enhanced darkfield microscopy (EDFM) and hyperspectral imaging (HSI) to easily and rapidly image nanoparticles in tissues from toxicology studies and map the distribution of nanoparticles throughout biological samples based on elemental composition.
|
|
“The current gold standard for visualization of nanoparticles in tissue samples is electron microscopy, which is highly time- and resource-intensive,” said Dr. Sara Brenner, Assistant Professor of Nanobioscience and Assistant Vice President for NanoHealth Initiatives at SUNY Poly and corresponding author of the study. “Availability of an alternative, rapid, and cost-effective method would relieve this analytical bottleneck, not only in nanotoxicology, but in many fields where nanoscale visualization is critical. The time and cost burdens associated with nanomaterial analysis and characterization in complex media are hindering progress in many research disciplines.”
|
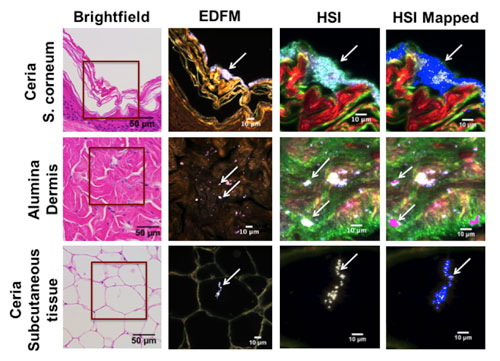 |
| Porcine skin tissue exposed to ENM-containing solution and mapped against the reference spectral library (RSL). Rows correspond to porcine skin tissue exposed to ceria and alumina ENMs, respectively. Each column shows the same field of view imaged with different techniques. The first column corresponds to a brightfield image of a hematoxylin and eosin stained sample (40x magnification). The area enclosed in a red square was magnified to 100x and viewed using EDFM (column 2) and HSI (column 3), where ENMs appear as high contrast elements (arrows). Column 4 shows the HSI image mapped against the RSL, where the positive matches are shown in blue for ceria and in magenta for alumina. From top to bottom, the rows correspond to: stratum corneum, dermis, and subcutaneous tissue, respectively. (click on image to enlarge)
|
|
As nanoparticles are increasingly incorporated into industrial processes and consumer products, studying the potential effects of exposure is critical to ensure the health and safety of workers, consumers, and the environment. The semiconductor industry utilizes metal oxide nanoparticles in a fabrication process, which has been identified by the industry as a critical area for health and safety research due to the potential for worker exposure. In their recent publication, the researchers were interested in visually locating metal oxide nanoparticles in an ex vivo porcine skin tissue model of cutaneous exposure.
|
|
“Nanomaterials have been used for decades in the dermatology consumer space, ranging from sunscreens to anti-aging cosmetics to antimicrobial dressings. Our ability to dispel concerns regarding safety has been limited due to the constraints of our imaging approaches, which is why the publication of this now validated technique is so important,” said Dr. Adam Friedman, Associate Professor of Dermatology and Director of Translational Research at the George Washington School of Medicine and Health Sciences.
|
|
The research team utilized CytoViva, Inc.’s HSI system, which incorporates an enhanced darkfield microscope that has improved contrast and a high signal-to-noise ratio for easy visualization of nanoparticles, as well as a hyperspectral imaging camera, which combines spectrophotometry and imaging, using advanced optics and algorithms to capture a spectrum from 400-1,000nm at each pixel in a hyperspectral image. Hyperspectral data can then be used to identify materials of interest in a sample without the need for fluorescent labeling or other destructive sample preparation techniques.
|
|
Not only did the researchers demonstrate the capability of EDFM-HSI to identify and map metal oxide nanoparticles in a porcine skin tissue model of exposure (Figure 1), but they also confirmed this method using traditional methods: Raman spectroscopy (RS) and scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDS) for elemental analysis.
|
|
After identifying areas within positive control tissue samples that were known to contain nanoparticles of interest, the same areas were analyzed via SEM-EDS and RS, which confirmed the identity of the materials. Once these areas were confirmed to be the nanoparticles of interest, reference spectral libraries (RSLs) containing hyperspectral data were created from these areas.
|
|
RSLs were then used to map the experimental samples to assess the presence and distribution of nanoparticles in those tissues, using the spectral angle mapper (SAM) algorithm in the hyperspectral imaging and analysis software (ENVI 4.8).
|
|
Although EDFM-HSI has lower spatial resolution than electron microscopy, it confers several advantages over traditional electron microscopy and Raman spectroscopy methods, particularly in terms of time- and cost-reduction, making it an attractive alternative for the visualization and identification of nanoparticles. Since EDFM-HSI is useful for studying nanoparticle biodistribution, it can be also be applied in areas of medical research, such as nanoscale drug delivery. The research team is expanding this work to other toxicological models of exposure, various nanomaterials, and other media types, including environmental and occupational exposure samples.
|
|
In partnership with the National Institute for Occupational Safety and Health (NIOSH), EDFM-HSI methods are being developed to analyze air filters worn by workers who handle engineered nanomaterials, which reveals information about inhalation exposure in the workplace. Further work also continues on protocol development to assess nanoparticle penetration in cutaneous exposure models and nanoparticle quantitation in histological samples. Notably, this research represents one of many focus areas in the NanoHealth & Safety Consortium (NHSC), a SUNY Poly-based public-private platform linking human and environmental health and safety across industries to promote nanotechnology commercialization, which is a key component of a recently announced White House initiative.
|
|
“New and emerging analytical methods and tools for nanomaterial detection, visualization, and characterization must keep pace with innovation in terms of nanomaterial development, use, and commercialization. Forms of higher-throughput screening and direct visualization technology, such as this one, can be leveraged for studying not only nanomaterial behavior in biological systems, but also applied in the context of exposure assessment. The system has great versatility and high practical utility – we’ve only begun to scratch the surface of what it can do,” said Dr. Brenner.
|

