| Posted: Jul 20, 2016 |
Garnet-type fast ionic conductor for all-solid-state lithium battery
(Nanowerk News) Rechargeable all-solid-state lithium batteries are expected to be one of the next-generation energy storage devices because of their high energy density, safety, and excellent cycle stability. The materials used for the solid electrolyte must not only have a high lithium-ion conductivity above 1 mS/cm at room temperature, but also possess chemical stability.
|
|
Oxide-based solid electrolyte materials have several advantages over sulfide-based ones, such as their chemical stability and ease of handling. Conversely, the formation of a solid-solid interface with low resistance between the solid electrolyte and the electrode is another challenging issue in achieving better electrochemical performance in solid-state batteries with an oxide-based SE.
|
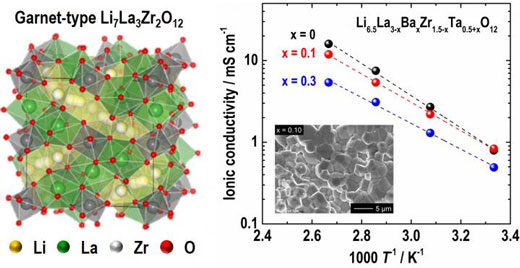 |
| Fig. 1 shows crystal structure of cubic garnet-type Li7La3Zr2O12 (LLZO, left) and temperature dependence of ionic conductivity for Ba- and Ta-substituted LLZO with different compositions. (Image: Toyohashi University of Technology)
|
|
In this study (Frontiers in Energy Research, "Development of Lithium-Stuffed Garnet-Type Oxide Solid Electrolytes with High Ionic Conductivity for Application to All-Solid-State Batteries"), Ryoji Inada and his colleagues at the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, developed a garnet-type, fast ionic conducting oxide as the solid electrolyte for an all-solid-state battery. Using the developed material, the rechargeable all-solid-state battery was fabricated and tested.
|
|
The research team investigated the influence of alien cation (Ba2+ and Ta5+) substitution in Li7La3Zr2O12 (LLZO, Fig. 1) on the crystal phase, microstructure, and ionic conducting property systematically. In order to stabilize the highly conductive cubic garnet phase, the Li concentration in the chemical formulae was fixed at 6.5 so that the formula of the compound may be expressed as Li6.5La3-xBaxZr1.5-xTa0.5+xO12 (LLBZTO).
|
|
As a result, the highest room temperature conductivity of 0.83 mS/cm was obtained in LLBZTO garnet with Ba and Ta contents of 0.1 and 1.6, respectively (Fig. 1). The Activation energy of LLBZT garnet tended to decrease monotonically with an increasing Ba substitution level; however, the excess Ba and Ta substitution degraded the conductivity.
|
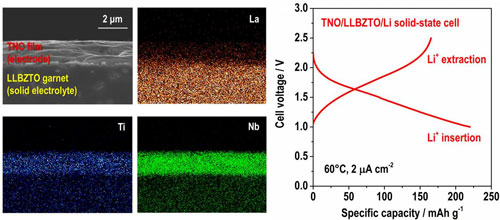 |
| Fig. 2 shows cross sectional SEM image and corresponding elementary distribution of Ti, Nb, and La for TNO film electrode formed on LLBZTO garnet (left). Charge and discharge curves for TNO/LLBZTO/Li all-solid-state battery tested at 60ºC (right). (Image: Toyohashi University of Technology) (click on image to enlarge)
|
|
In addition, they confirmed that LLBZTO garnet has a wide electrochemical potential window, hence, various positive and negative electrode materials can potentially be utilized to construct an all-solid-state battery. We fabricated a TiNb2O7 (TNO) film electrode on LLBZTO using the aerosol deposition method and demonstrated its charge and discharge reaction using a TNO/LLBZTO /Li all-solid-state battery sample (Fig. 2).
|
|
These results indicate that the developed LLBZTO garnet can be used as a solid electrolyte in an all-solid-state battery and contribute to the realization of a very safe rechargeable battery for large-scale power sources, even though additional investigation is needed in order to enhance the performance of solid-state batteries. The researchers carried out further study to realize solid-state batteries with high energy density.
|


