| Posted: Aug 03, 2016 |
Novel porous materials made from flexible 'spaghetti-like' molecules
(Nanowerk News) Known for their extraordinary surface area, a sugar packet equivalent of metal-organic framework provides a city block (2.5 acres) of area to absorb gas or catalyze reactions. The challenge comes in consistently manufacturing these frameworks, known as MOFs.
|
|
Scientists changed our understanding of MOFs. They uprooted the belief that these frameworks must be made from rigid starting materials. Their new design strategy transformed flexible one-dimensional molecular chains, called polymers, into three-dimensional porous structures by incorporating the flexible polymers into the synthesis of MOFs ("polyMOFs: A class of interconvertible polymer-metal-organic-framework hybrid materials").
|
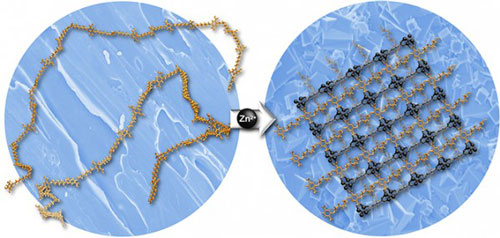 |
| Usually, flexible polymers pack together to form dense materials without pores. However, this new design strategy combines charged metal atoms (zinc ions, Zn2+, in the above example) with molecular chains (polymers) to create an entirely new hybrid material with a cage-like structure. The material has a three-dimensional rigid structure that is highly porous and regular in all directions. This moisture-resistant material has potential applications in storing natural gas or hydrogen for advanced vehicles, capturing carbon dioxide, separation, manufacturing, and purifying chemicals.
|
|
This new design strategy produces a material that advantageously combines the structural integrity of MOFs and the processability of polymers. The result is novel, tunable porous materials. These materials could advance a range of separation and storage technologies, including capturing industrially emitted, climate-changing carbon dioxide; storing compressed natural gas for utility trucks and taxis; and storing hydrogen for environmentally-friendly fuel cell vehicles.
|
|
It was believed that the scaffolding nature of MOFs could only be prepared from rigid starting materials. The preparation of such porous materials from one-dimensional polymers is challenging because flexible polymers tend to pack closely together, resulting in dense, disordered material without pores.
|
|
Now, researchers at the University of California-San Diego have demonstrated the remarkable transformation of non-porous polymers with metal ions into three-dimensional highly porous, crystalline hybrid MOF materials. In this new design strategy, the scientists heated polymer chains with metal ions to allow ordering and assembly into a hybrid MOF material.
|
|
The polymers end up bound to the metal ions. This coordination creates a porous scaffolding in the hybrid MOF. This new design strategy allows for facile tuning of the resulting porous material. Scientists can tune the hybrid MOF by changing the chemistry of the polymer, the length of its molecular chain, or the processing temperature.
|
|
Scientists varied the resulting morphology from spheres to cubes, to films. These hybrid MOFs combine the structural integrity and porosity needed for gas separation and storage applications with the advantageous processability of polymers. Some of the hybrid MOFs demonstrated enhanced carbon dioxide uptake that could specifically advance carbon capture technologies.
|

