| Posted: Aug 05, 2016 |
Ice dielectric response studies find preparation is key
(Nanowerk News) Despite numerous studies on the dielectric properties of water ice, a discrepancy in the results of different studies had remained unexplained for decades. Now a collaboration of researchers at Tokai University in Japan show that the differences likely arise as a result of the ice preparation conditions and resulting impurity levels (J. Phys. Chem. B, "Dielectric Relaxation Time of Ice-Ih with Different Preparation").
|
|
In 1952 Auty and Cole published results from studies of water ice’s dielectric relaxation time, τice – the lag in the response of molecules to changes in the electric field. They found a linear “Arrhenius” relationship between τice and temperature. However in 1981 Johari and Whalley reported observed changes in the relationship at 230 K. Subsequent experiments using various parameters – thereby excluding possible explanations based on crystal size or a metastable crystal surface - have also noted temperatures at which the dielectric behaviour apparently crosses over to a new regime.
|
|
The conundrum also affects studies of proteins and glycerol dissolved in ice. As Naoki Shinyashiki and colleagues explain in their report, “An explanation of the mechanism underlying the difference between the τice (Auty-1952) and τice (Johari-1981) would enable the use of τice observed in aqueous systems to be utilized effectively not only for understanding ice dynamics but also as a probe of the dynamic viscoelastic behaviour of water and solute molecules through ice growth.”
|
|
Kaito Sasaki, Rio Kita, Naoki Shinyashiki, and Shin Yagihara prepared ice for the study in three different ways and found that the agreement with Auty et al. or Johari et al. depended on the preparation process. Ice that was slow-cooled using a magnetic stirrer to avoid bubbles and rapid crystallization replicated the results of Auty et al down to the lowest temperatures considered in the 1952 study. The researchers suggest this is caused by the lower level of impurities for ice prepared this way.
|
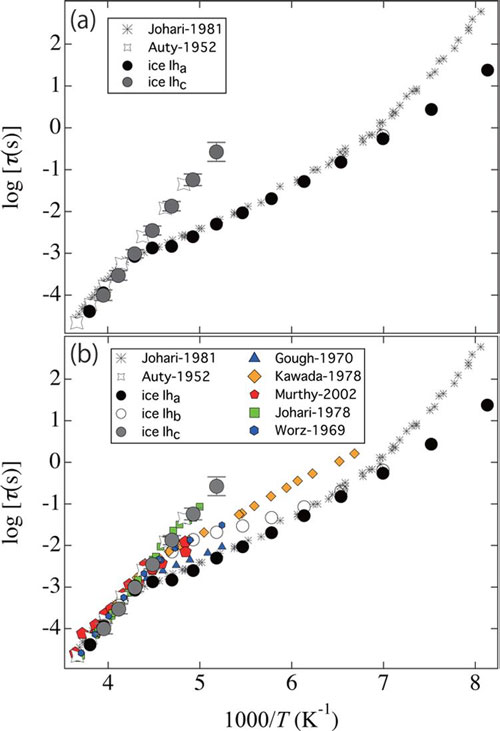 |
| Temperature dependences of relaxation times of ice-Iha (cooled at 5 K per minute), ice-Ihb (cooled at 265 K for 5 h), and ice-Ihc (cooled at 265 K for 5 h with a magnetic stirrer to further prevent rapid crystallization and bubble formation). For comparison, relaxation times obtained by Johari in 1981 (black star, D2O) and in 1978 (green square), Auty (open gray star), Murthy (red pentagon), Gough (purple triangle), Kawada (orange diamond), and Wörz (blue hexagon) are plotted together. For the ease of viewing, the top panel (a) shows the relaxation times of ice-Iha and ice-Ihc together with that of ice obtained by Johari in 1981 (black star) and Auty (open gray star) in 1952. Error bars for ice-Ihc are given by standard deviation with four times measurements.
|
|
Background
|
|
Dielectric relaxation
|
|
A dielectric material is an electrical insulator that can be polarized by an electric field. The delay in its response to the electric field is the dielectric relaxation time.
|
|
The simplest mathematical description for this relaxation process – the Debye equation as set out by Peter Debye in 1913 - describes the molecules as identical dipoles. Modifications to the equation have been developed since, including those by Debye himself with Erich Hückel in 1923, which allows for ionic solutes, whereby the perfect dipole approximation breaks down.
|
|
Alongside Kenneth S Cole, Robert H Cole – co-author with Auty in the 1952 paper on the dielectric properties of ice - set forth the modified Cole-Cole formulations of dielectric behaviour that incorporate broadening or dampening in the response time. This Cole-Cole behaviour is observed in the results reported here by the Tohai group.
|
|
The Arrhenius relationship
|
|
Svante Arrhenius proposed the Arrhenius relationship in 1889 to describe the relationship between rates of reaction and temperature. The relationship suggests a linear relationship, such that reaction rates double with every 10 K increase in temperature.
|
|
Arrhenius explained the relationship with the concept of an activation energy – the minimum energy required for reactants to form a product. The concept of an activation energy also applies to dielectric properties of activation as reported in the Cole-Cole temperature-dependent behaviour reported here.
|
|
Arrhenius relationships were observed in ice for all the three preparation techniques described by the researchers at Tohai University, although below a certain temperature they crossover into another temperature-dependent dielectric regime. For ice slow-cooled with a magnetic stirrer this crossover is not observed down to temperatures below that reported by Auty et al. The researchers suggest this is due to the absence of impurity-produced orientational defects.
|
|
Proteins in ice
|
|
Proteins are normally found in aqueous environments. In all studies of the dielectric properties of proteins dissolved in aqueous ice an unknown relaxation process is observed within a similar frequency range. Understanding the dielectric properties of ice can help to understand the contributions to the dielectric response of protein solutions and their viscoelastic properties.
|

