| Posted: May 30, 2017 |
Nanosubmarine with self-destroying activity
(Nanowerk News) Autonomous targeting and release of drugs at their site of action are desired features of nanomedical systems.
|
|
Now, a team of Dutch scientists has designed a nanomotor that has these functions: An antitumor drug encapsulated in self-propelled, self-assembled stomatocytes is carried across the cellular membrane and released inside the cell upon a chemical redox signal that disassembles the vesicle membrane.
|
|
This deliver and unpack nanomedicinal system is introduced in the journal Angewandte Chemie ("Redox-Sensitive Stomatocyte Nanomotors: Destruction and Drug Release in the Presence of Glutathione").
|
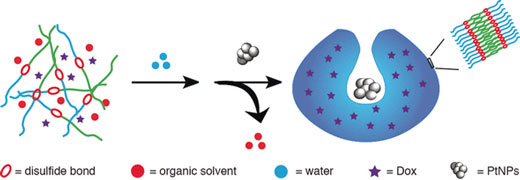 |
| Self-destroyed redox-sensitive stomatocyte nanomotor delivers and releases drugs for cells. (© Wiley)
|
|
Self-propelling nanovesicles are attractive transport vehicles for drugs. If they are fueled by hydrogen peroxide, these vesicles can take up directed motion responding to its concentration gradient. Combining the ideas of self-propelling nanomotors, drug encapsulation, and triggered destruction of the nanocarrier, Daniela A. Wilson and her team at Radboud University, The Netherlands, have designed an artificial self-propelling vesicle, which is sealed by a block copolymer shell and opens to release the loaded drug load if it encounters higher concentrations of glutathione, a chemical signal inside cells.
|
|
Glutathione is a so-called redox molecule, an antioxidant. In the cell, this small peptide acts as a scavenger of reactive oxygen species; besides, it serves as a pool for the amino acid cysteine. Elevated levels of glutathione are frequently found inside tumor cells.
|
|
Wilson and her team came upon glutathione in their attempt to find a door-opener for their drug-loaded, self-propelling artificial vesicles: "The small glutathione can enter into the PEG shell of the nanomotor and then break down the redox-responsive disulfide bonds [...], resulting in cleavage of the outside PEG shell," they wrote.
|
|
Thus, upon cleaving disulfide bonds, glutathione triggers the vesicle membrane disassembly, and the content of the vesicle, which can be a drug, is distributed in the target cell.
|
|
The material of the vesicle membrane is a block copolymer made of poly(ethylene glycol) (PEG) and polystyrene, both of which are connected by a disulfide bond. During self-assembly, a hydrophilic anticancer drug can be encapsulated. Then, the artificial vesicle is transformed into a bowl-shaped stomatocyte, a vesicle with a special dent or groove, by adding the engine, platinum nanoparticles.
|
|
This nanoparticle catalyst degrades hydrogen peroxide, which is typically produced by tumor cells, propelling the stomatocytes forward, for example, across the cell membrane. There, glutathione, as it were, presses the door handle, opens the vesicle, and stops the motion by catalyst poisoning.
|
|
For human cell cultures, the authors demonstrated internalization of the stomatocyte nanomotors, their degradation, and drug release. They propose the nano-submarine as an attractive concept for future drug delivery applications.
|

