| Posted: Jul 11, 2017 |
Breakthrough tool predicts properties of theoretical materials
(Nanowerk News) Scientists at the University of North Carolina at Chapel Hill and Duke University have created the first general-purpose method for using machine learning to predict the properties of new metals, ceramics and other crystalline materials and to find new uses for existing materials, a discovery that could save countless hours wasted in the trial-and-error process of creating new and better materials.
|
|
Researchers led by Olexandr Isayev, Ph.D., and Alexander Tropsha, Ph.D., at the UNC Eshelman School of Pharmacy used data on approximately 60,000 unique materials from the National Institute of Standards and Technology's Inorganic Crystal Structure Database to create a new methodology they call Properties Labeled Materials Fragments.
|
|
Using machine learning to analyze and model existing crystal structures, the PLMF method is able to predict the properties of new materials proposed by scientists and engineers. The tool was even able to fill in missing values for properties of materials in the NIST database that had never been tested experimentally.
|
|
"Technology is often driven by the discovery of new materials, but the process of discovering these materials has always been rather haphazard," Tropsha said. "Out new tool applies the data- and knowledge-driven approach we use in the pharmaceutical sciences to design drugs. Because creating new materials takes an incredible amount of time and effort that often ends in disappointment, our PLMF tool allows materials scientists to test a new idea before they even lift a finger to synthesize it."
|
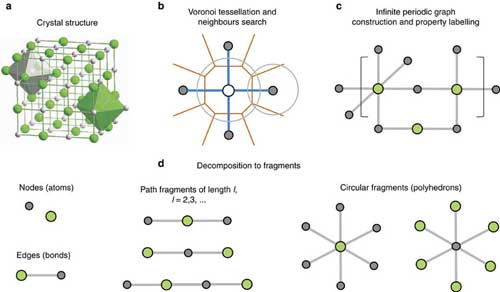 |
| The crystal structure (a) is analysed for atomic neighbours via Voronoi tessellation (b). After property labelling, the resulting periodic graph (c) is decomposed into simple subgraphs (d). (© Nature Publishing Group) (click on image to enlarge)
|
|
Tropsha is the K.H. Lee Distinguished Professor at the School and director of the Laboratory for Molecular Modeling. Isayev is a research assistant professor. Their work was published in Nature Communications ("Universal fragment descriptors for predicting properties of inorganic crystals"), and the PLMF tool is publicly available as a user-friendly web application at http://alfow.org/aflow-ml.
|
|
The PLMF method works by creating "fingerprints" from the structure of the crystals that comprise the smallest units of inorganic materials like ceramics, metals and metal alloys. Combining the fingerprints with machine learning allowed the creation of universal models capable of accurately predicting eight critical electronic and thermomechanical properties of virtually any inorganic crystalline material. The properties include conductivity, stiffness and compressibility, heat transfer and response to temperature change, and the team plans to incorporate more properties as they collect more data, Isayev said.
|
|
"In many practical projects, people know the range of values they want for a particular property," Isayev said. "We can leverage what we know about these materials and savvy machine learning to rapidly screen potential materials for the right property. Researchers can quickly narrow candidate materials and avoid many extraneous and complex calculations. This saves money, time and computational resources."
|
|
In the first practical application for the machine learning, the team worked with Assistant Professor Jim Cahoon, Ph.D., in the UNC Department of Chemistry to design a new electrode material for a type of low-cost solar cells. The currently used nickel oxide, is not very efficient, toxic and requires organic solvents to work in the cell.
|
|
Scientists virtually screened 50,000 known inorganic compounds and identified lead titanate as the most promising material and subsequent testing confirmed it. The devices using lead titanate exhibited the best performance in aqueous solution, allowing a switch away from solvents to a water-based solution that could help drive down costs while being more environmentally friendly.
|
|
"Lead titanate likely would not have been the first choice of most materials scientists because its structure is so dissimilar to nickel oxide," Isayev said. "Materials derived from iron, cobalt or copper would be more likely to be considered because they are more chemically similar to nickel. The PLMF and machine learning found a simple and novel solutions that saved untold hours of trial-and-error searching."
|

