| Posted: May 08, 2017 | |
The complex interplay of nanosilver and wheat roots(Nanowerk News) Scientists have hypothesized that silver nanoparticles (Ag-NPs) may act as “Trojan horses” entering living organisms and then releasing Ag+ over time, causing toxicity. This has been recently proposed as the mechanism by which Ag2S-NPs could be toxic to wheat and cowpea. |
|
| New work by a team of French scientists, just published in Environmental Science & Technology ("Silver Nanoparticles and Wheat Roots: A Complex Interplay"), evidences a more complex scheme – although the Trojan horse scenario is very likely to take place. | |
 |
|
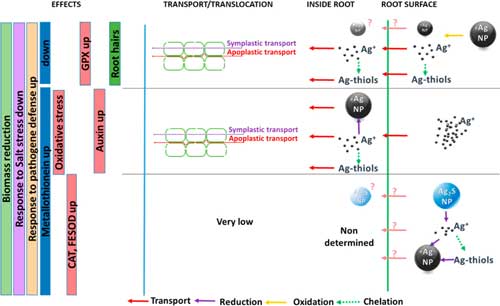
| Synthesis of the observed changes in silver speciation that take place inside and outside wheat roots depending upon the starting materials, proposed transfer pathways, and induced effects on plants. (© ACS) (click on image to enlarge) | |
| As illustrated in the figure above, interconversions of silver forms outside and inside the plant, leading to a mixture of silver species, were observed. | |
| The distribution of silverspecies probably evolves over time, leading to a complex exposition pattern for plants, also exposed to HS- in the case of Ag2S-NPs. | |
| The authors write that these conflicting results usually seen in the literature concerning the toxicity of silver nanoparticles are generally ascribed to differences in exposure conditions, structural properties of nanoparticles, and plant species. This evolving silver speciation may represent an additional source of variability and conflicting results. | |
| Data from this study showed drastic different responses of the plant depending upon the starting nanoparticles, high-lighting the importance to include transformed nanoparticles in ecotoxicological studies. | |
| Although the uptake and translocation of silver was lower for the Ag2S-NPs exposure compared to the Ag-NP exposure, phytotoxicity symptoms were observed. | |
| According to the research team, these results show that the sulfidation of Ag-NPs is not a perfect antidote to toxicity and that Ag2S-NPs are not as stable as expected when exposed to plant roots. | |
| In agricultural soils, the rhizospheric activity of plants might partly dissolve Ag2S, causing some impacts on crop quality and yield and, more generally, on ecological services. |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
