| Posted: Jun 06, 2017 | |
Dual-action cancer nanomedicine therapy(Nanowerk News) A French-Australian research team has fabricated antibody-coated porous silicon nanoparticles that can actively target cells through binding to specific cell-surface receptors. They demonstrated that these nanoparticles can bind to and selectively deliver multiple therapeutics to human B cells in vitro. |
|
| By co-loading a chemotherapy drug with gold nanoclusters, the system took advantage of combined chemotherapy and hyperthermia therapy and provided synergistic effects in eradicating targeted cells. | |
| In particular, as the team points out, they have highlighted the ability of gold nanoclusters to affect cells by an externally applied µW field, boosting the cytotoxicity of the co-loaded anticancer drug campthotecin. | |
| These results have been published in Small ("Dual-Action Cancer Therapy with Targeted Porous Silicon Nanovectors"). | |
| Porous silicon nanoparticles (pSiNPs) are biodegradable, suitable for conjugation with targeting moieties, and excellent carriers of chemotherapy drugs. In this work, the scientists utilized these unique properties of porous silicon nanoparticles and loaded them with two different therapeutics, while immobilizing cell-specific antibodies to ensure active targeting. | |
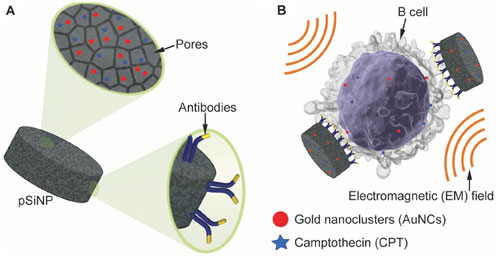 |
|
| A) Illustration of the highly porous pSiNPs that can load the chemotherapeutic camptothecin and gold nanoclusters, and immobilize targeting antibodies. B) Schematic of their ability to selectively deliver the payload to human B lymphocytes and enhance the cytotoxicity when exposed to an electromagnetic field. (© Wiley-VCH Verlag) (click on image to enlarge) | |
| By encapsulating a chemotherapy drug and gold nanoclusters into the antibody-coated pSiNPs, the researchers illustrate the potential of this system to combine localized hyperthermia and chemotherapy. | |
| "Our platform could allow significant enhancement of localized cytotoxicity in tumors by an external µW field," the authors conclude in their paper. |
| Source: Wiley | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
