| Posted: Jul 11, 2017 | |
Selectively killing bacteria with magnetic nanoparticles(Nanowerk News) Based on previous investigations of nanoparticles as effective antibacterial coating ingredients, scientists at Clemson University hypothesized that multianchored magnetic nanoparticles conjugated with sialic-acid moieties that mimic host-cell receptors specific for Escherichia coli strain K99 (EC K99) adhesins would induce rapid clustering of EC K99 in the presence of these nanoparticles, and when such bacteria-nanoparticles aggregates are exposed to an alternating magnetic field (AMF), it would result in enhanced and selective inactivation/killing of EC K99. |
|
| Reporting their findings in Advanced Functional Materials ("Multianchored Glycoconjugate-Functionalized Magnetic Nanoparticles: A Tool for Selective Killing of Targeted Bacteria via Alternating Magnetic Fields"), they demonstrated proof-of-concept multianchored glycoconjugate (Neu5Ac(α2-3)-Gal-(β1-4)Glcβ-sp) GM3-magnetic nanoparticles (GM3-MNPs) that have high affinity to adhesin of EC K99. | |
 |
|
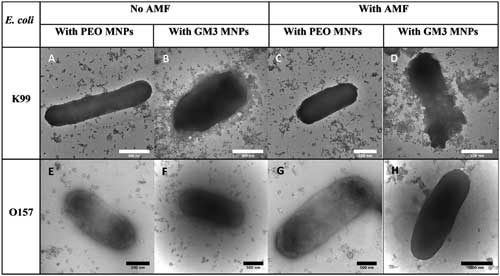
| TEM images of GM3-MNPs induced bacterial membrane damage of E. coli strains: A,B,E,F) before AMF, and C,D,G,H) after AMF treatment for 120 min. Concentration of MNPs: 650 µg Fe mL-1. Scale bar is 500 nm. (click on image to enlarge) | |
| The team's nanoparticle system can specifically interact with adhesin molecules of EC K99 and cause agglutination through nanoparticle–bacteria complex. Applying treatment with an alternating magnetic field to such complex caused significant reduction in viability of targeted bacteria EC K99 in both pure-culture and mixedcultures settings due to possible highly localized temperature increase. | |
| Exposure to such conditions resulting in compromised membrane integrity of EC K99. Moreover, GM3-MNPs coupled with AMF resulted in significant decrease in the overall intracellular ATP levels of the bacterium. | |
| These results demonstrate that the unique multianchored nanoparticle system in the presence of AMF can be effectively used as novel nonantibiotic platform for local and selective inactivation of the target bacteria in biological systems without affecting the viability of nearby cells/tissues. | |
| This study therefore serves as proof-of-concept that a high degree of selective bacterial killing can be obtained without using traditional antibiotics. | |
| In the event of gastrointestinal tract infections caused by enterotoxigenic E. coli pathogens, administered antibiotics can disrupt/destroy beneficial gut microflora in addition to pathogens. It could cause various side effects in the human body along with giving rise to antibiotic-resistant bacterial strains. | |
| The Clemson team's system can find useful applications in treating such infections in animals and humans and in conditions when administered antibiotics, especially those of the last-line-of-defense drugs, fail to eradicate the infections due to drug-resistance. | |
| "Future studies will involve optimization of particle parameters including nanoparticle core-size and polymer coatings, as well as a detailed investigation on the effects of field strength and frequency to maximize killing rate of clinically relevant multidrug resistant bacterial pathogens," the authors conclude their report. "Additionally, biocompatibility of our nanoparticle system will be evaluated in human cell-lines and small animal models." |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
