| Posted: Jul 14, 2017 | |
Controlling complex nanostructures with critical Casimir forces(Nanowerk News) In new work, reported in Advanced Materials ("Switching Colloidal Superstructures by Critical Casimir Forces"), researchers from The Netherlands show that the application of critical Casimir forces on multivalent patchy particles indeed allows fine control over the assembly of colloidal superstructures. |
|
| Tunable site-specific critical Casimir interactions provide novel control to assemble complex colloidal superstructures. Their temperature dependence offers direct in situ adjustment of the bond stiffness, allowing the mechanical properties of the assembled structure to be tailored and switched between different morphological states. | |
 |
|
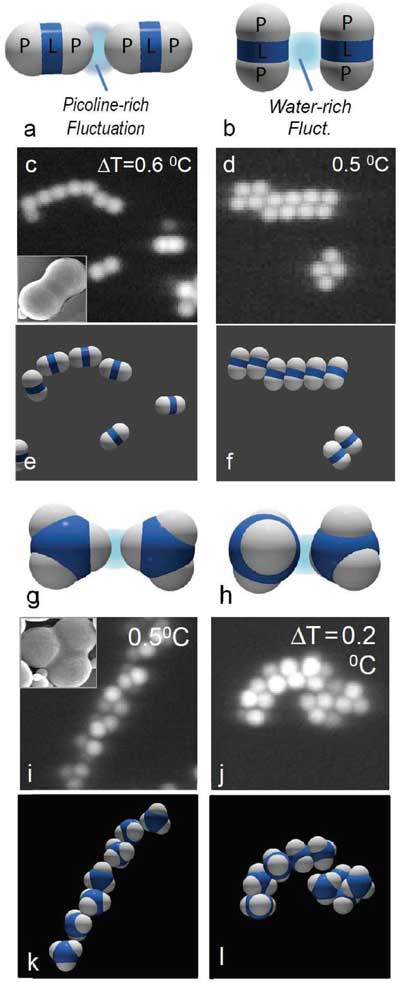
| Bonding of dimer and trimer particles by critical Casimir forces. a–f) Bonding of dimer particles in 3MP-poor (left) and 3MP-rich solvents (right). a,b) Principle of the specific critical Casimir interaction: 3MP-rich fluctuations confined between hydrophobic patches (P) cause patch-to-patch binding for solvent compositions c3MP < cc (a), while water-rich fluctuations confined between hydrophilic shells (L) lead to shell-to-shell binding for solvent compositions c3MP > cc. (b). c,d) Confocal microscope images and e,f) schematic of resulting particle configurations. Chain-like structures in solvents with c3MP = 0.25 < cc demonstrate patch-to-patch binding (c,e), while parallel structures in solvents with c3MP = 0.31 demonstrate sideways attraction (d,f). The inset in panel (c) shows scanning electron microscope (SEM) image of dimer particle. g–l) Bonding of trimer particles in 3MP-poor (left) and 3MP-rich solvents (right): schematic (g,h), confocal microscope images (i,j) and schematic of particle configurations (k,l). Staggered chains indicate patch binding (i,k), while curled filaments indicate shell binding (j,l). (© Wiley-VCH Verlag)) | |
| The team demonstrates specific and adjustable critical Casimir bonding of hydrophobic and hydrophilic particle patches with in situ control over bond energy, range, and bond stiffness. | |
| As they report in their paper, they assembled dimer particles into colloidal analogues of molecular polymers with adjustable bending stiffness, which we measure directly from thermally activated bending fluctuations. | |
| These colloidal polymers exhibit a collapse transition close to the solvent critical point, reminiscent of molecular polymer collapse when solvent conditions change from good to poor. | |
| Using Monte Carlo simulations with an optimized potential based on experimentally measured pair correlation functions, the team shows that the colloidal chain collapse results from the growing interaction range due to the increasing solvent correlation length close to the solvent critical point. | |
| "We demonstrate that this experimental control applies also to particles with higher valence such as trimers and tetramers, allowing the assembly of even more complex, switchable structures," the authors conclude. "These results open new routes to the in situ control of nanostructures with actively controllable mechanical properties and morphologies." |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
