| Posted: April 10, 2009 |
A first-of-a-kind switch in chemical bonding by a zirconium atom spotted by scientists |
|
(Nanowerk News) A zirconium atom can switch easily between two different bonding patterns in an unusual molecule created by Japanese scientists.
|
|
The molecule’s odd behavior is the first example of this particular type of chemical bonding shift, according to Noriyuki Suzuki of RIKEN's Advanced Science Institute in Wako, and his colleagues at Saitama University and Saitama Institute of Technology. “These complexes have very unique structures and show interesting movement,” says Suzuki. An example is shown in Figure 1.
|
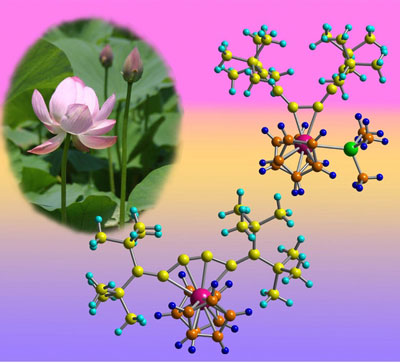 |
| Schematic showing that adding or removing an extra chemical group (green) to the zirconium atom (pink) can open and close the structure of the molecule like the petals of a lotus flower.
|
|
The team discovered the phenomenon when they were experimenting with a molecule (hexapentaene) made from a chain of six carbon atoms, all doubly bonded to each other. The chain is surrounded by a long cloud of delocalized electrons, which can bond with a zirconium-based compound to create a new complex.
|
|
Once the new complex has formed, the zirconium atom normally tends to bridge between carbon atoms in different parts of the chain, creating a five-membered ring. But when very bulky groups were added to each end of the chain, the zirconium switches its allegiance so that it sticks to just the central carbon–carbon double bond.
|
|
The complex could be toggled between its two bonding modes by adding or removing other chemical groups such as phosphines around the zirconium atom. Further experiments showed that this sort of shift was the first step in a reaction the scientists had previously studied, where adding an isocyanide chemical to the zirconium complex created a compound with a small ring of four carbon atoms at its heart.
|
|
This switching behavior is well known in certain ring-shaped organic molecules, but is much rarer in these molecular chains, and is unprecedented with this particular compound. “It suggests the possibility of a molecular motion like scissors or tongs,” says Suzuki. The research is published in the Journal of the American Chemical Society (Reversible haptotropic shift in zirconocene–hexapentaene complexes).
|
|
Suzuki’s team has spent several years investigating a range of such zirconium complexes, which were long assumed to be too unstable to isolate (Isolation and Structural Characterization of 1-Zirconacyclopent-3-yne, Five-Membered Cyclic Alkynes and Transformation of a 1-Zirconacyclopent-3-yne, a Five-Membered Cycloalkyne, into a 1-Zirconacyclopent-3-ene and Formal 1-Zirconacyclopenta-2,3-dienes).
|
|
Although there are no immediate applications for this family of complexes, Suzuki suggests that it may be possible to use the scissoring action identified in their latest research to capture another molecule or ion. “We might be able to achieve a molecular machine that catches a certain target,” says Suzuki.
|

