| Posted: April 24, 2009 |
The color and the shape |
|
(Nanowerk News) As with higher-level organisms, reproduction is among the primary biological imperatives of the cell. This process is regulated through a multi-stage cell cycle; the more rapidly this cycle proceeds, the more quickly division takes place. A stalled cycle may be indicative of terminal cell differentiation, while rapid cycling might denote either healthy tissue growth or tumor formation, depending on the context.
|
|
The ‘Fucci’ method developed by Atsushi Miyawaki and colleagues at the RIKEN Brain Science Institute in Wako offers a powerful tool for monitoring cell cycle procession (Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression). Fucci uses two fluorescently tagged proteins, Cdt1 and Geminin, that are differentially expressed throughout the cell cycle as a visual indicator of cell cycle phase. Cdt1 is predominantly expressed in the G1 phase, in which cells idle prior to S-phase DNA replication; as replication begins, Cdt1 levels drop and Geminin levels rise, indicating onset of active cell division.
|
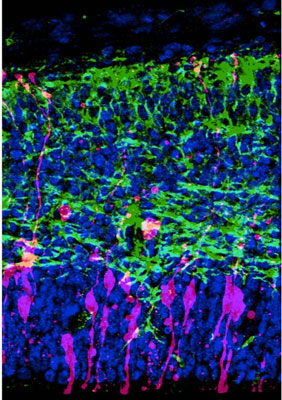 |
| Demonstration of the modified Fucci method in neural progenitor cells within the developing cerebral cortex of a mouse. This image contains superimposed fluorescent signals from a modified Geminin derivative that localizes to both nucleus and cytoplasm (pink), the neuronal cell marker Tuj1 (green), and a generic stain for cell nuclei (blue).
|
|
Fucci has proven effective in numerous applications, but Miyawaki’s team ran into complications while studying the differentiation of neuronal progenitors. “The nuclear targeting of the fluorescence signal did not permit us to identify cell types and differentiation states by their characteristic morphologies,” explains Miyawaki. To address this, his team developed a Fucci variant that illuminates the cytoplasm, enabling simultaneous monitoring of transitions in cell shape and cell cycle state (Tracing the Silhouette of Individual Cells in S/G2/M Phases with Fluorescence).
|
|
Normally, Geminin is strictly localized to the nucleus; however, by trimming a segment from the end of the protein, Miyawaki’s team was able to engineer Fucci-ready variants that linger in the cytoplasm. Upon testing their new Fucci markers in neural progenitors in the developing mouse brain, they found that they could now classify different types of neuroepithelial progenitors based on changes in growth and morphology as well as their cycling behavior.
|
|
For Fucci to maintain an accurate readout, the cell must degrade labeled Geminin at the end of each cycle, and Miyawaki’s team found that this requires the protein to be in the nucleus. Fortunately, the modified Geminin is only transiently located in the cytoplasm before being shuttled back to the nucleus, minimizing the overall impact on Fucci’s effectiveness.
|
|
Miyawaki’s future goals include expansion of the Fucci palette to incorporate additional color tags and spotlight different, specific segments of the cell cycle, but he sees immediate value in this latest variant. “This type of indicator, which traces the silhouette of individual cells with fluorescence in a cell cycle-dependent manner, will be useful for imaging mitotic cells with interesting shapes, including neural progenitor cells,” he says.
|

