| Posted: June 26, 2009 |
Taking proteins for a ride |
|
(Nanowerk News) Cells produce thousands of proteins that are essential for life, but the proteins are of no use unless they can be delivered to the right places. Now, Ken Matsuoka and co-workers at the RIKEN Plant Science Center in Yokohama, Kyushu University in Fukuoka and Niigata University have discovered a subcellular structure in plants that carries proteins and glycans to the correct locations, especially outside of the cell ("A Mobile Secretory Vesicle Cluster Involved in Mass Transport from the Golgi to the Plant Cell Exterior").
|
|
The newly identified delivery structure arises from another substructure in the cell called the Golgi apparatus. If one imagines a cell as a factory producing proteins, the Golgi apparatus can be thought of as the sorting office, where proteins are organized and packaged into bundles ready for their journey around the body.
|
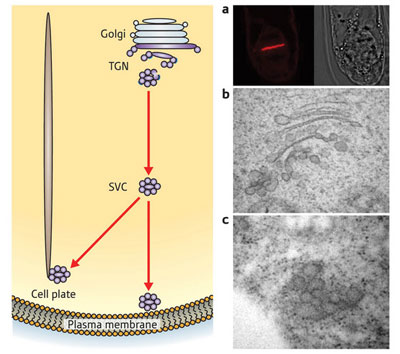 |
| Figure 1: Secretory vesicle clusters (SVCs) emerge from the Golgi apparatus in plant cells. They move either to the plasma membrane to secrete proteins and glycans, or to the cell plate where they are involved in cell division. On the right are microscope images of (a) the cell plate, (b) the Golgi apparatus and (c) an SVC.
|
|
The protein bundles, packed together with lipids, are called transport vesicles. One of their functions is to travel to the cell membrane and secrete proteins from the cell—a process called exocytosis.
|
|
Plants, in particular, have complicated ‘post-Golgi’ compartments that influence vesicles during the last stages of exocytosis. “It is not yet clear whether these compartments are the sole elements in the late secretory pathway of plants, how they interact, or how they are involved in exocytosis,” says Matsuoka.
|
|
Matsuoka and co-workers monitored the movement of vesicles in tobacco plant cells, by fluorescent tagging of a known vesicle protein called secretory carrier membrane protein 2 (SCAMP2). They found that SCAMP2 accumulates in the Golgi network, but not in known post-Golgi compartments. Instead, it appears in clusters of between 5 and 12 vesicles, each around 50 to 100 nanometers in diameter.
|
|
|
|
The researchers named these new structures ‘secretory vesicle clusters’, or SVCs. The SVCs can move separately from the Golgi network, and are often seen tethered to cell walls, where they are probably involved in secreting proteins and glycans from the cell.
|
|
Furthermore, the SVCs appear to play an important role in cell division. SVCs in dividing cells were targeted towards the cell plate—a thick wall of glycans and proteins that forms down the centre of a cell before the cell splits in two (Fig. 1).
|
|
The researchers found SVCs in Arabidopsis and rice plants as well as tobacco. Therefore the SVCs represent a standard delivery mechanism supplying cells with the necessary ingredients for maintaining life.
|
|
“We are now isolating the SVCs to analyze their constituents,” says Matsuoka. “[Then] we will be able to analyze the molecular mechanisms of SVC transport and for tethering vesicles together in SVCs.”
|

