| Posted: August 14, 2009 |
A tight fit helps energy transmit in artificial photosynthetic systems |
|
(Nanowerk News) Although plants have efficiently captured energy from sunlight for millions of years, producing light-harvesting and energy storage devices based on photosynthesis is no easy task. Now, a research team led by Makoto Fujita from the University of Tokyo and Tahei Tahara from the RIKEN Advanced Science Institute has found a simple way to mimic the initial stage of photosynthesis by mechanically trapping a guest molecule inside a cage structure (Energy Transfer in a Mechanically Trapped Exciplex).
|
|
Prototypical artificial photosynthetic systems contain donor- and acceptor-type molecules. When light is absorbed by the donor, it becomes photo-excited—its electrons move to higher energy states. The acceptor group can receive and store these energetic electrons, but only if the donor and acceptor come together into what is known as an exciplex, or an excited state complex.
|
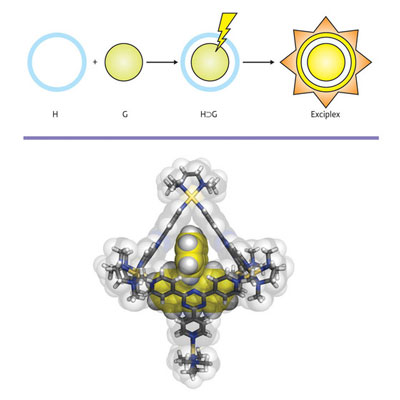 |
| Figure 1: Schematic formation of a mechanically trapped host (H)–guest (G) exciplex complex (top) and the x-ray crystal structure of the host–guest complex (bottom).
|
|
The difficulty is bringing together the donor and acceptor groups. An exciplex can form only if the two components are close enough and in the proper orientation during photo-excitation.
|
|
Fujita and Tahara’s team ensured exciplex formation by locking a photoactive donor molecule called bisanthracene inside a molecular cage acceptor (Fig. 1). The self-assembled cage is highly water soluble as it contains six charged palladium atoms. The cage panels, however, are organic molecules and form a hydrophobic (water-repelling) pocket inside the cage when dissolved in water.
|
|
According to Jeremy Klosterman, the lead author of the study, the donor molecule bisanthracene is not soluble in water and, at high temperatures, is driven into the hydrophobic cage pocket. Once the solution cools, the bisanthracene is too large to exit the cage and remains trapped inside.
|
|
|
|
“Synthetically, our system is incredibly straightforward,” says Klosterman. “Simply mixing the host cage and the guest bisanthracene in water and heating causes the exciplex to self-assemble.”
|
|
Ultrafast laser spectroscopy of the host–guest complex found that the excited bisanthracene donor transferred the majority of its energy, 82%, to the exciplex state. Klosterman says the effective energy transfer is due to the extremely tight fit and strong interactions between the mechanically linked host and guest.
|
|
“This study helped us resolve an important question,” states Klosterman. Typically fluorescent molecules are non-emissive upon encapsulation by cages, but now they can infer that energy transfer into the host–guest exciplex state decreases the fluorescence lifetime.
|
|
By choosing a guest molecule that does not form an exciplex, the researchers have developed a new water-soluble fluorescent dye with a long lifetime—ideal for applications including biological sensing and imaging.
|

