| Posted: September 25, 2009 |
Pores for thought |
|
(Nanowerk News) A porous coordination polymer (PCP) that strongly adsorbs methanol, a model guest molecule, has been prepared by Masakazu Higuchi from the RIKEN SPring-8 Center in Harima and co-workers from the University of Kyoto, the Japan Synchrotron Radiation Research Institute and Osaka Prefecture University ("Porous Coordination Polymer with Pyridinium Cationic Surface, [Zn2(tpa)2(cpb)]").
|
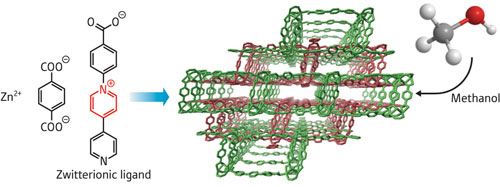 |
| Schematic showing the continuous porous structure (center), which can adsorb methanol, formed by reaction of zinc ions (Zn2+) with terephthalic acid and 1-(4-carboxyphenyl) – 4,4’-bipyridinium ligands (left).
|
|
The new material is important because porous materials that can adsorb guest molecules offer opportunities in finding ways to store hydrogen fuel, and to sequester waste gas such as carbon dioxide, which can reduce the impact of burning fossil fuels. Porous coordination polymers (PCPs) provide a particularly attractive option in both endeavors because they contain micropores and their surfaces can be designed to have specific properties.
|
|
Also known as metal–organic frameworks (MOFs), PCPs are formed between metal ions—often from transition metals such as zinc—and well-defined organic ligands that can bond to more than one metal atom. With sufficiently rigid ligands, such that a single ligand cannot just coordinate to a single metal ion, it is possible to produce a continuous network of metal ions held together by the ligands. It is within the pores of these PCPs that guest molecules such as gases can be accommodated.
|
|
For guest molecules to be adsorbed efficiently they must interact with the pore walls. “We thought that electrostatically charged walls would be beneficial, but this introduced a new problem,” explains Higuchi, “the overall structure must be electrically neutral and the counter-ions required to achieve this occupy the pores of the PCP meaning that they are blocked to guest molecules.”
|
|
Higuchi and colleagues’ PCP is based on the coordination of zinc ions with a zwitterionic ligand, which is electrically neutral, but carries separated positive and negative charges (Figure above). They showed that guest molecules of methanol adsorb more strongly than a similar PCP made with uncharged ligands. It can also adsorb more guest molecules because the pores are not blocked by counter-ions.
|
|
|
|
The zwitterionic ligand used in the new material described by Higuchi and his colleagues means that the pore walls are highly charged but additional counter-ions are not required. They have also shown that the material adsorbs methanol more strongly than a similar PCP with uncharged pore walls. “In the future, we plan to investigate how other guest molecules interact with the charged pore surface” says Higuchi. “Ultimately, we hope to see this develop into a material that can be made on an industrial scale.”
|

