| Posted: December 23, 2009 |
Tracing the traces - nano-concentrations of a toxic compound detected in chlorinated tap water |
|
(Nanowerk News) Drinking water can transmit a number of diseases, including typhoid, dysentery, cholera, and diarrhea, which can then spread explosively throughout an entire service area. To avoid this problem, drinking water must be disinfected. After treatment and disinfection, the water is usually safe. To manage the disease risk until it reaches the tap, most waterworks throughout the world use chlorine or chlorine-containing chemicals for disinfection. Beneficial though the chlorination of water may be, it does have one potential drawback: studies have suggested that there may be a connection between the ingestion of chlorinated tap water and an increased risk of bladder cancer.
|
|
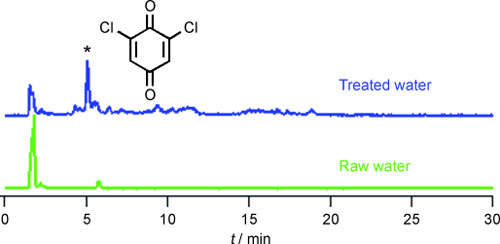
Scientists at the University of Alberta in Canada have now revealed a chlorination by-product of great interest: As the team led by Xing-Fang Li reports in the journal Angewandte Chemie ("Single-Molecule Investigations of a Photoswitchable Nanodevice"), they were able to detect traces of the toxic compound dichloroquinone.
|
 |
|
Chlorination has been use to disinfect water for decades. Through reactions with natural organic molecules in the water, it can lead to formation of trace amounts of toxic by-products, such as chloroform and halogenated acetic acid derivatives. The maximum allowed concentrations of these substances were legally regulated some years ago. Newer studies have suggested that these substances are not likely to pose a cancer risk. Instead, other possible by-products, such as halogenated quinones, which may be present in treated water at previously undetectable concentrations, are now under suspicion. Quinones are six-membered carbon rings with two oxygen atoms bound by double bonds to opposite ends of the molecule, and they occur in some microorganisms. Quinones that also contain halogen atoms such as chlorine or bromine may react with DNA and proteins at very low concentrations, causing damage to organisms.
|
|
The Canadian team has now been the first to successfully identify a representative of this class of compounds, 2,6-dichloro-1,4-benzoquinone, in chlorinated drinking water. To accomplish this, the researchers had to develop a special analytical procedure based on liquid chromatography (LC), electrospray ionization (ESI), and tandem mass spectrometry (tandem-MS). In actual water samples, they used this technique to detect this compound in quantities of a few nanograms per liter of water. The toxicology of some chloroquinones indicates that they could pose a risk of bladder cancer.
|

