| Posted: January 6, 2010 |
Cancer biology: Breaking the cycle |
|
(Nanowerk News) Cell division is an essential biological process, but careful oversight is necessary to ensure that normal proliferation doesn’t spin out of control into cancerous growth. Accordingly, while transcription factor E2F1 is capable of activating numerous genes that move the cell cycle forward, it is generally kept inactive via interaction with repressor protein pRb. When conditions are appropriate for cell division, kinase enzymes chemically modify pRb, disrupting the pRb-E2F complex and enabling E2F-mediated transcription.
|
|
Many cellular pathways receive additional coordination from microRNAs—short RNA molecules that target specific genes for downregulation. However, fully characterizing the function of these various microRNAs poses a serious challenge, says Qiang Yu of A*STAR’s Genome Institute of Singapore. “A microRNA can target several hundred genes, and we can only focus on functional targets relevant to a microRNA’s function,” he says. “My lab has been interested in the E2F1-regulated gene network for years and, given the important role of microRNAs in regulating transcriptional networks, we started to look at microRNA regulation of the E2F1 pathway.”
|
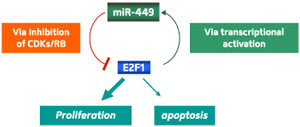 |
| Fig. 1: A diagram illustrating the role of miR-449a/b as a check to cell division. These microRNAs downregulate kinases that activate E2F1, effectively constraining the oncogenic role of E2F1 in driving cell proliferation. The induction of these microRNAs by E2F1 constitutes a negative feedback mechanism to control E2F1 activity. (Reproduced, with permission, from Feng, M. & Yu, Q. miR-449 regulates CDK-Rb-E2F1 through an auto-regulatory feedback circuit. Cell Cycle 9, 1–2 (2010) © 2010 Landes Bioscience)
|
|
Yu and his co-workers designed a screen that enabled them to identify microRNAs whose production is stimulated by the activation of E2F1, revealing two likely candidates: miR-449a and miR-449b ("miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb–E2F1 activity through a feedback loop by targeting CDK6 and CDC25A"). Both microRNAs are encoded from a single genetic locus, and the researchers confirmed the presence of two E2F1 binding sites within this gene’s regulatory sequences.
|
|
MicroRNAs work by directly binding to RNA transcripts with complementary sequences, and by analyzing a gene database, Yu’s team was able to identify the CDK6 and CDC25A genes as likely targets for inhibition by miR-449a/b. Both genes encode kinases that alleviate pRb repression, giving these results important clinical implications. “CDK6 and CDC25A are oncogenes, often overexpressed in human cancer,” says Yu. “This suggests a feedback mechanism controlling E2F1 activity that is lost in cancer cells due to repressed expression of miR-449a/b.”
|
|
This hypothesis was supported by data from a broad array of cancer cell lines; for several of these, coercing the cells into expressing miR-449a/b proved sufficient to bring their uncontrolled proliferation to a halt. This model also suggests that these microRNAs ultimately reduce their own gene activity by driving E2F1 inactivation—a useful strategy for keeping miR-449a/b levels under tight control (Fig. 1).
|
|
Yu believes these findings could guide the development of new weapons against a variety of cancers. “It will be interesting to see if miR-449a/b can be exploited as a therapeutic agent, because of its efficient tumor suppressor function,” he says.
|

