| Posted: January 27, 2010 |
Electron microscopic examination of Alzheimer's amyloid fibrils |
|
(Nanowerk News) Alzheimer’s disease, Parkinson’s disease, type-II diabetes, and prion diseases like BSE all involve the deposition of amyloid fibrils in tissues and organs. These are fibrous clumps of incorrectly folded proteins; their exact structures and their roles in pathological processes are not yet completely understood.
|
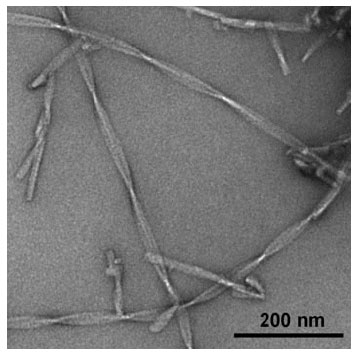 |
| Versatile nanomaterial: Unusually high nanoscale flexibility was displayed by amyloid fibils in electron microscopy studies (see picture). This finding is relevant for understanding amyloid pathogenicity and for potential biotechnological applications.
|
|
By using electron microscopic images of flash frozen samples, researchers have now been able to examine the exact structure of Alzheimer’s amyloid fibrils and to assess their mechanical properties. As the team reports in the journal Angewandte Chemie ("Nanoscale Flexibility Parameters of Alzheimer Amyloid Fibrils Determined by Electron Cryo-Microscopy"), the fibrils are very stiff—one of the underlying causes of their pathogenicity.
|
|
Because amyloid fibrils are very difficult to analyze with traditional biophysical techniques, Marcus Fändrich (Max Planck Unit for Enzymology of Protein Folding, Halle/Saale, Germany), Carsten Sachse (MRC Laboratory of Molecular Biology, Cambridge, UK), and Nikolaus Grigorieff (Brandeis University, Waltham, USA) were forced to take another approach: They examined Alzheimer’s amyloid fibrils by electron cryomicroscopy. “These experiments allowed us to examine the structure of the fibrils at a previously unattainable resolution,” explains Fändrich.
|
|
The fibrils appear in twisted bands about 20 nm wide and are often bent in the raw electron microscopic images. “These bent fibrils are a snapshot of the fibrils in solution,” says Fändrich. “We use the degree of bending and twisting to calculate how stiff the fibrils are.” This revealed that the Alzheimer’s amyloid fibrils are relatively rigid structures. “The uncontrolled formation of such stiff fibrils is presumably critical for the pathogenicity of amyloid fibrils,” reports Fändrich. “In many amyloid diseases, the fibrils are preferentially deposited in tissues that are normally contractile or elastic, like the heart muscle or the walls of blood vessels. Medical findings indicate that the fibrils somewhat stiffen these tissues.”
|
|
“In addition, our data may help to better evaluate the possible uses of amyloid fibers as novel biotechnological agents,” reports Fändrich. Based on their material properties and ease of modification, amyloid fibers are potentially interesting as novel building materials.
|

