| Posted: February 5, 2010 |
A simple method for high-purity separation of metallic and semiconducting carbon nanotubes |
|
(Nanowerk News) On November 27, the New Energy and Industrial Technology Development Organization (NEDO) and the National Institute of Advanced Industrial Science and Technology (AIST) announced that, as a part of Industrial Technology Research Grant Program, Takeshi Tanaka (Research Scientist) of AIST, and his colleagues have successfully developed a method for high-purity separation of single-walled carbon nanotubes (SWCNTs) Note 1) into metallic SWCNTs and semiconducting SWCNTs by using a column packed with agarose gel Note 2). Since this method allows low-cost separation by automation of the separation process and by permitting reuse of the gel, industrial applications of separated metallic and semiconducting SWCNTs utilizing their characteristics are expected.
|
|
This result were published in Applied Physics Express by he Japan Society of Applied Physics and will be published at nano tech 2010, which will be held on February 17 to 19, 2010, at Tokyo Big Sight.
|
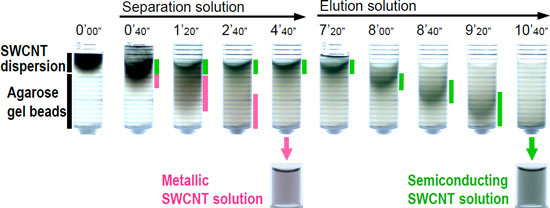 |
| Figure 1 Separation of metallic and semiconducting SWCNTs in a column packed with agarose gel beads. When SWCNTs dispersed in a solution were applied to the column, reddish metallic SWCNTs passed through the gel (pink bar), while green semiconducting SWCNTs (green bar) were adsorbed onto the gel. The adsorbed semiconducting SWCNTs can be collected with the eluent. (The numerals indicate the time in minute’ second”.)
|
|
Note1) A single-walled carbon nanotube is a tubular substance in which carbon atoms form hexagonal networks similar to that observed in graphite. The diameter of the tube is about 0.7–4 nm (1 nm = one billionth of a meter). The tube exhibits metallic or semiconducting properties according to the arrangement of hexagons.
|
|
Note2) Agarose is a polysaccharide obtained from seaweed and is the principal ingredient of agar. Agarose gel is prepared by dissolving agarose in hot water and cooling the solution until it solidifies.
|
|
Summary
|
|
Takeshi Tanaka (Research Scientist) and Hiromichi Kataura (Leader), the Self-Assembled Nano-Electronics Group, the Nanotechnology Research Institute (Director: Nobutsugu Minami) of AIST (President: Tamotsu Nomakuchi), developed a method for high-purity separation of SWCNTs into metallic SWCNTs and semiconducting SWCNTs by using a column packed with agarose gel beads, as a part of Industrial Technology Research Grant Program of NEDO.
|
|
The synthesis of SWCNTs usually yields a 1:2 mixture of metallic and semiconducting SWCNTs. Although these two types of SWCNTs must be separated for their application in electrical devices, this separation cannot be achieved easily. AIST had developed methods for separating metallic and semiconducting SWCNTs by using agarose gel. AIST has subsequently improved these methods and achieved a low-cost high-purity separation method. This method involves the use of a column packed with agarose gel beads, in which the semiconducting SWCNTs are selectively adsorbed and then eluted (Fig. 1). The gel-packed column can be used repeatedly, and the separation process can be easily automated. This approach allows low-cost production of SWCNTs, thereby facilitating industrial production of metallic and semiconducting SWCNTs.
|
|
1. Social Background for Research
|
|
Depending on the arrangement of the carbon atoms, SWCNTs exhibit metallic or semiconducting properties. Synthesis of SWCNTs usually yields a mixture of these two types. If the two types of SWCNTs can be separated with high purity, they can be employed for many potential applications. Instead of the transparent conductive materials prepared using rare metals, metallic SWCNTs would be used as transparent electrodes in liquid crystal displays or solar cells. Semiconducting SWCNTs would be used as transparent and flexible transistors. High-integration high-speed SWCNT computers may be realized by using metallic SWCNTs in wiring and semiconducting SWCNTs in transistors in the future.
|
|
To date, no method is available for independently synthesizing the two materials, which have different electrical properties, and many attempts have been performed to isolate individual materials from mixtures. However, due to problems associated with yield, purity, and cost, none of the proposed methods has been employed for mass production. Therefore, a low-cost separation method that can be employed for mass production is being sought.
|
|
2. Outline and importance of research
|
|
AIST had reported that electrophoresis using SWCNT-containing agarose gel could efficiently separate metallic and semiconducting SWCNTs and provide a high yield of SWCNTs; furthermore, the two types of SWCNTs could be separated from the SWCNT-containing agarose gel without application of an electric field. These studies have been developed further to develop a superior separating method.
|
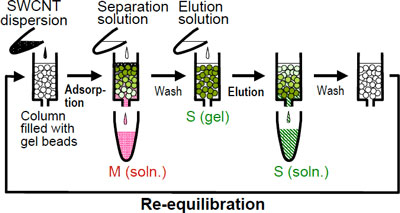 |
| Figure 2: A schematic illustration of the separation of metallic and semiconducting SWCNTs using a gel-packed column.
|
|
In agarose-gel-based separation methods for metallic and semiconducting SWCNTs developed by AIST before, separation and recovery of the semiconducting SWCNTs adsorbed onto the gel involved melting of the gel. Moreover, the purity of the SWCNTs separated using these methods was not satisfactory. To overcome these shortcomings, we employed column chromatography in the gel-based separation method (Fig. 2). When a SWCNT dispersed solution was applied to a column packed with agarose gel beads and a solution for separation was passed through the column, the metallic SWCNTs were collected with the solution for separation, while the semiconducting SWCNTs were adsorbed onto the gel. After washing out the metallic SWCNTs remaining in the column, the semiconducting SWCNTs adsorbed onto the gel were eluted with a solution containing suitable surfactants (Fig. 1). The column packed with agarose gel can be used again after equilibration. Moreover, the separation performance of the column was not deteriorated by repeated use (Fig. 3a). In comparison with the samples separated using the previous electrophoresis or conventional centrifugation methods for SWCNT-containing gels, the purity of the semiconducting and metallic SWCNTs obtained using our novel approach improved from 95% and 70% to 95% and 90%, respectively (Fig. 3b). These purity values were comparable to those of the samples obtained by density-gradient ultracentrifugation, which can separate samples with high purity but is inferior in terms of efficiency and cost.
|
|
In the previous separation methods performed using SWCNT-containing gels, it was thought that the purity of the obtained SWCNTs decreased because of the wide distribution of length of the SWCNTs; owing to the network (sieve) structure of the gel, separation should be influenced by electrical properties as well as the length of the SWCNTs. In contrast, in the column method, adsorption and desorption occur mainly on the surface of the gel; therefore, it is thought that the network structure has little influence on the separation and that SWCNTs are separated primarily according to the differences in their electrical properties. Thus, the new method can provide high-purity separation.
|
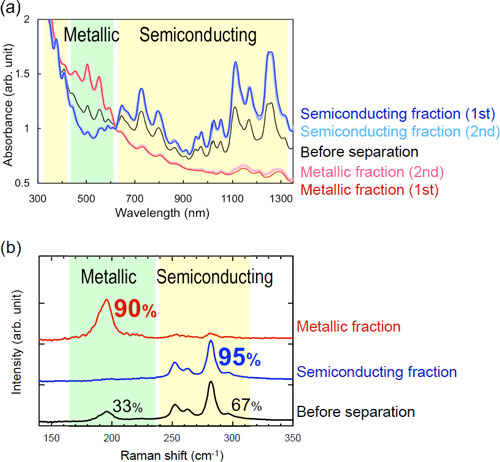 |
| Figure 3: (a) Absorption spectra of the separated SWCNTs obtained by the first and second separations using the same gel-packed column (b) Raman scattering spectra of the SWCNTs obtained by the first separation
|
|
The peaks in the green zone are assigned to metallic SWCNTs and the peaks in a yellow zone are assigned to semiconducting SWCNTs.
|
|
Column chromatography is a widely used separation technique in the industrial processes for production of chemical products and pharmaceuticals. Therefore, techniques for automation of separation and large-scale separation have been already established, and there are almost no obstacles preventing the introduction to industrial production.
|
|
The new separation method can yield samples with purity comparable to that of the samples obtained by density-gradient ultracentrifugation, which is a high-purity separation technique. Further, because of the repeated use of gel-packed columns and the lower labor costs due to automation, the separation cost in the new method would be less than one-tenth of that of density-gradient ultracentrifugation. Thus, this approach will allow supply of inexpensive, high-purity metallic and semiconducting SWCNTs.
|
|
3. Future Schedule
|
|
Further studies on the preparation of the solution in which the SWCNTs are dispersed will be performed to improve the preparation efficiency and cost efficiency of this step. In addition, research on large-scale separation of metallic SWCNTs and semiconducting SWCNTs will be promoted in cooperation with companies, and the applications of the separated SWCNTs will be exploited.
|



