| Posted: March 4, 2010 |
Technique to probe hidden dynamics of molecular biology |
|
(Nanowerk News) Funded by a $1 million grant from the W.M. Keck Foundation, University of Chicago scientists are aiming to develop a reliable method for determining how biological processes emerge from molecular interactions. The method may permit them to “rewire” the regulatory circuitry of insulin-secreting pancreatic beta cells, which play a major role in type-2 diabetes.
|
|
“Despite the enormous amount of study directed at diabetes, there’s really very little understanding of the collective mechanisms that govern or regulate insulin secretion,” said project director Aaron Dinner, Associate Professor in Chemistry.
|
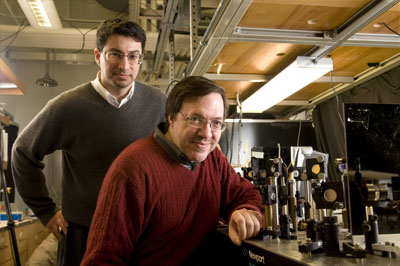 |
| Aaron Dinner and Norbert Scherer are collaborating with UChicago colleagues Rustem Ismagilov and Louis Philipson to elucidate the molecular dynamics underlying insulin secretion in pancreatic beta cells.
|
|
A second goal: to control cell behavior and function more generally, which may ultimately culminate in other applications, including the bioremediation of environmental problems. Collaborating with Dinner on the project are Louis Philipson, PhD’82, MD ’86, Professor in Medicine and Director of the University of Chicago Kovler Diabetes Center; and chemistry professors Rustem Ismagilov and Norbert Scherer, SB’82.
|
|
The four scientists share an interest in the collective behavior of cells that emerges from a complex ensemble of atoms and molecules working in concert at different scales of time and space. “In a living system you have this hierarchy of coupled time and length scales,” Dinner said. “How is it that all of these different dynamics at one time and length scale get coupled to dynamics at another scale?”
|
|
The collaborators have worked together previously in various pairs. “It seemed natural to put those different pair-wise interactions together,” Dinner said.
|
|
Philipson and Scherer, for example, worked together to pioneer a microscopy method for imaging activity inside beta cells that led up to insulin secretion under different conditions. Ismagilov and Philipson collaborated on a means of efficiently measuring and analyzing beta-cell secretions. And Dinner and Scherer have analyzed the dynamics of an oddly behaving RNA molecule.
|
|
Non-intuitive molecular behavior
|
|
Dinner and Scherer’s study revealed some non-intuitive, hidden dynamics. They experimented with the molecule in solution, expecting it to move slowly, somewhat like a person walking around in a swimming pool. But after changing the chemical solution they found that the molecule behaved in a non-intuitive way.
|
|
“It was as though something was driving it,” Dinner said.
|
|
The chemical pulses they had introduced into the molecule’s watery environment were the driving force of the dynamic oscillations they observed. In their next step, they applied the process to a bacterium, coupling cycles inside the cell that would ordinarily operate on different time scales. The scientists then analyzed the bacterium’s response to the chemical pulses for insights into its internal properties.
|
|
The similar use of optical, magnetic and spectroscopic techniques is a standard means of probing molecular dynamics. Based on their RNA research, Scherer and Dinner realized that a chemical version of the technique might provide a whole new way of studying cellular dynamics. They call their new technique “chemical perturbation spectroscopy.”
|
|
“We measure everything at a single-cell level so we can quantify in detail what each single cell is doing as it evolves through multiple generations,” Scherer said. “These studies are allowing us to lay the groundwork for how to measure perturbations that we apply to cells, and how to do the analysis. Essentially, none of this has been done before, so we have to invent the approach.”
|
|
Once the details are worked out, Scherer said, “We expect to be able to target certain cell functions and, let’s say, increase insulin output from the beta cells.”
|

