| Posted: July 7, 2010 |
Producing clean hydrogen by controlling lithium-water reaction |
|
(Nanowerk News) Haoshen Zhou (Leader) and Yonggang Wang (Post-Doctoral Research Scientist) of Energy Interface Technology Group, the Energy Technology Research Institute of the National Institute of Advanced Industrial Science and Technology (AIST), have developed the concept of a clean hydrogen production system based on controlled lithium-water electrochemical reactions and have successfully investigated the system.
|
|
In recent years, hydrogen has attracted attention as a clean energy source for reducing the increase in CO2 emissions from the burning of fossil fuels. However, the use of hydrogen as an energy source involves many issues. It is particularly necessary to establish technologies for the safe and convenient storage of hydrogen. To achieve this safety and convenience it is desirable to produce the needed hydrogen on-site.
|
|
The concept of a device called a lithium-water battery, using metallic lithium as the active material at a negative electrode and water as the active material at a positive electrode, has been proposed before, but the use of hydrogen, a byproduct of the battery, had not been investigated. We have now developed a new concept of producing both hydrogen and electricity by stably controlled reactions using metallic lithium as the negative electrode and carbon as the positive electrode, with a hybrid electrolyte (a combination of an organic electrolyte, a solid electrolyte, and an aqueous electrolyte) (see Figure). We have succeeded in the substantiation of the concept with this system, which allows as much clean hydrogen to be produced as needed and when needed, while generating electricity from electrical discharge from the electrochemical reactions. The amount of hydrogen produced is currently about 230 µmol h-1 per one square centimeter of positive electrode surface. This system can be regenerated by recharging, and it can therefore be used as an energy storage system that stores electrical energy from natural energy sources such as solar cells, and surplus power at night in the form of metallic lithium; it is thus able to produce hydrogen and electricity as needed. We intend to investigate suitable applications for this system.
|
|
The results of this research will be published in ChemSusChem, a German academic journal.
|
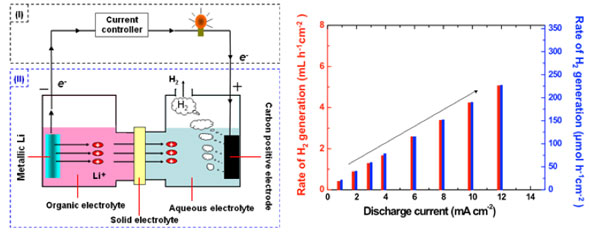 |
| Left: Schematic of the lithium-water battery and hydrogen production. Right: Amount of hydrogen produced at the positive electrode of the lithium-water battery.
|
|
Social Background of Research
|
|
With increasing CO2 emissions from the heavy use of fossil fuels and sharply changing oil prices, the effective use of hydrogen energy is attracting attention as part of a strategy to build a sustainable low-carbon society. However, to create a hydrogen society (i.e. a society that uses hydrogen as a major energy source) we need to establish innovative hydrogen production and energy storage technologies.
|
|
History of Research
|
|
In the process of development of lithium-ion batteries, the Energy Technology Research Institute of AIST has demonstrated that high power density batteries can be realized by using nanostructured materials for the electrodes. In order to increase energy density of batteries, we have conducted research on a lithium-air battery and a lithium-copper secondary battery that allow recycling of lithium.
|
|
In focusing on the appllication of lithium-water electrochemical reactions using the hybrid electrolyte, we have conceived a system of producing hydrogen while generating electricity in accordance with energy demand.
|
|
Details of Research
|
|
The newly developed system is based on the concept of the hybrid electrolyte used in the lithium-air battery and the lithium-copper secondary battery. In this system, metallic lithium is used as the active material for the negative electrode and water for the positive electrode, and carbon is used as the positive electrode current collector. A solid electrolyte that allows only lithium ions (Li+) to pass through is used as the separator between the organic electrolyte on the negative electrode side and the aqueous electrolyte on the positive electrode side. This configuration prevents mixing of the organic and aqueous electrolytes and allows control of the lithium-water electrochemical reactions, because hydrogen ions (H+) or hydroxide ions (OH-) do not reach the organic electrolyte.
|
|
The following reactions occur at the electrodes during electrical discharge:
|
|
1) Reaction at the negative electrode: Li -> Li+ + e-
|
|
Metallic lithium (Li) is dissolved in the organic electrolyte as lithium ions and electrons (e-) are supplied to the wire. The lithium ions pass through the solid electrolyte and move to the aqueous electrolyte on the positive electrode side.
|
|
2) Reaction at the positive electrode: 2H2O + 2e- -> 2OH- + H2 (gas)
|
|
Electrons are supplied from the wire and the water as the active material is decomposed, producing hydrogen (H2) near the positive electrode current collector.
|
|
In this system, hydrogen is produced by the discharge reactions in the lithium-water battery. The hydrogen production rate can be controlled by regulating the discharge current. For discharge at a current density of 12 mA cm?2, the amount of hydrogen produced at the positive electrode current collector is presently about 5.2 mL h-1 cm-2 (about 230 µmol h-1 cm-2). The hydrogen production capacity would be increased some 10 times by increasing both the lithium ion conductivity of the solid electrolyte separator and the working temperature.
|
|
Lithium hydroxide (LiOH) as a product can be recovered by the reverse reaction of discharge, making it possible to reuse this system. Fluctuating electricity from renewable energy sources, such as wind and solar power, and surplus power at night can be stored in the form of lithium, and hydrogen and electricity can be produced by controlling the discharge current of the lithium-water battery according to demand. If solar or another power generation system is used, this system can be used as an energy storage and supply system for housing in areas without grid access.
|
|
Future Schedule
|
|
The hydrogen production system based on the controlled lithium-water reactions currently operates at the laboratory scale. We plan to improve the lithium-ion conductivity of the solid electrolyte and durability of the system for practical application. We also plan to study how to utilize the advantages of the system, such as its ability to supply both electricity and hydrogen and its ability to be regenerated by the recharging reaction, in order to contribute to the energy technology systems of the future.
|

