| Posted: Sep 17, 2010 |
|
Entangled frameworks limber up
|
|
(Nanowerk News) The quest to tune the three-dimensional (3D) molecular frameworks of materials called porous coordination polymers (PCPs) has taken a step forward thanks to a research team led by Ryotaro Matsuda and Susumu Kitagawa at the RIKEN SPring-8 Center in Harima and Kyoto University, Japan. The team, with members from Osaka Prefecture University, has described the influence of interpenetration of PCPs on the structural flexibility and gas sorption behavior of these materials, which show great potential for use in gas storage, heterogeneous catalysis and as separation materials ("Control of Interpenetration for Tuning Structural Flexibility Influences Sorption Properties").
|
|
The interpenetrated molecular frameworks of PCPs are composed of metal ions and bridging organic ligands. Materials scientists initially thought that interpenetration would reduce the available capacity of the voids within the structure. However, other researchers showed recently that such entangled structures exhibit high gas-uptake, as a result of increased internal surface area. Interpenetration also increases the thermal stability of flexible frameworks.
|
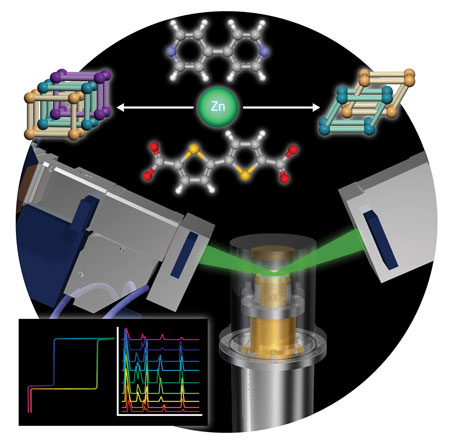 |
| Figure 1: Schematic representations of the synthesis of the two-fold and three-fold interpenetrated porous coordination polymers, structural analysis by x-ray powder diffraction and adsorption and desorption profiles.
|
|
These findings prompted Matsuda, Kitagawa and colleagues to make PCPs with the same chemical components but with either two-fold or three-fold interpenetration. Both forms of the 3D frameworks were made using a solvent templating method and were composed of zinc atoms and carboxylate- and pyridyl-based organic ligands (Fig. 1). The two forms allowed the researchers to test the correlation between various physical properties and the degree of entanglement of the polymers.
|
|
Crystal structure analyses of the two forms indicated that non-covalent interactions, namely π-π interactions, in the three-fold structure are more significant than in the two-fold structure. Consequently, the two-fold structure has a more flexible structure and is of lower thermal stability than the more rigid three-fold PCP.
|
|
Using coincident x-ray powder diffraction and adsorption measurements, the team also showed that the two forms of structures have completely different carbon dioxide (CO2) adsorption behavior. The two-fold structure can adsorb four times the amount of saturated CO2 than the three-fold structure, owing to its greater flexibility and dynamic capability. Sorption occurs as a stepwise progression as a result of crystallographic transformations triggered by the addition and removal of guest molecules.
|
|
"The next challenge is the control of adsorption properties by external stimuli such as light or magnetic field to realize on-demand gas separation and storage," says Matsuda. "This kind of material could be used to separate CO2 which is discharged from steelworks or to remove CO2 and hence keep air fresh in a spaceship."
|

