| Posted: Nov 05, 2010 |
|
'Prima donna' protein doesn't work well in pairs
|
|
(Nanowerk News) A new study by Rice University bioengineers finds that the workhorse proteins that move cargo inside living cells behave like prima donnas. The protein, called kinesin, is a two-legged molecular machine. Rice's scientists invented tools that could measure the pulling power of kinesin both singly and in pairs, and they report this week in Biophysical Journal that kinesins don't work well together -- in part because they are so effective on their own.
|
|
"Researchers have been investigating the mechanical properties of individual motor proteins for some time now, but this is the first time anyone's been able to tie a defined number of molecular motors to a cargo and watch them work together," said lead researcher Michael Diehl, assistant professor in bioengineering at Rice. "We know that more than one of these motors is attached to most cargoes, so understanding how they work together -- or fail to -- is a key to better understanding the intracellular transport system."
|
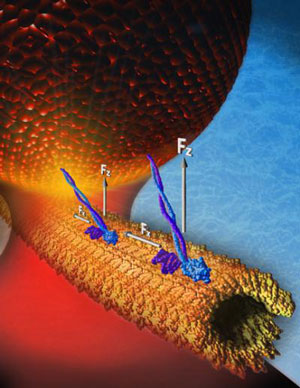 |
| Rice University researchers tethered pairs of kinesin motor proteins to plastic beads and measured their pulling power. They found the two-legged proteins, which are relatively fast and efficient on their own, had trouble keeping up with one another when they were connected.
|
|
Cargoes inside cells are hitched to teams of motor proteins and hauled from place to place like horse-drawn wagons. Like stagecoaches or wagons, many cargoes are pulled by several horses. But unlike a wagon, cellular cargoes often also have multiple teams pulling in opposite directions.
|
|
"Motor proteins move directionally," Diehl said. "They either move toward the cell's nucleus or they move away from the nucleus toward the periphery. Grouping different types of motors together allows cells to regulate cargo movement. But when there are multiple motors pulling antagonistically in opposite directions, what determines which group wins? What influences the balance? How do they cooperate or compete to get the right packages to the right place? Those are the kinds questions we're trying to answer."
|
|
Diehl said intracellular transport has become an increasingly hot topic over the past decade, in part because researchers have found that breakdowns in the transport system are linked to neurodegenerative diseases like amyotrophic lateral sclerosis (ALS) and Huntington's disease.
|
|
One question Diehl and lead co-authors Kenneth Jamison and Jonathan Driver helped answer in the new study is how much pulling power a pair of kinesins could apply to a cargo compared with the amount applied by a single kinesin.
|
|
The apparatus they created to study the problem was years in the making. Driver and Jamison, both graduate students, used strands of DNA to make a scaffold, a sort of molecular yoke that they could use to hitch a pair of kinesins to an experimental cargo. The cargo in their tests was a microscopic plastic bead. Using laser beams in an instrument called an optical trap, they attached teams of bead-pulling proteins to microtubule roadways.
|
|
As the motors walked down the road, they pulled the bead away from the center of the optical trap. At the same time, the lasers in the trap exerted counterpressure in an effort to move the bead back to the center of the trap. Eventually, the light won out, forcing the motors to let go and the cargo to snap back to the middle of the beam. By measuring the precise movements of the bead during this reaction, Diehl's team was able to determine exactly how much force a team of motors exerted on a bead.
|
|
"Compared with other motors, kinesin is actually a pretty strong performer," he said. "Single kinesin motor molecules can produce relatively large forces, and they rarely step in the wrong direction when walking along microtubules. This is remarkable behavior, considering kinesin is a molecular-scale machine that experiences significant thermal and chemical fluctuations."
|
|
Given how well they perform alone, it would be easy to assume that a group of kinesins would pull harder than a single kinesin. But Diehl points out that a team of kinesins can only harness the combined potential of both motors under certain circumstances.
|
|
"Our analyses show that the two kinesins must stay in close proximity to one another to cooperate effectively," he said. "Otherwise, one of the motors will tend to assume all of the applied force imposed on the cargo. Kinesin is relatively fast and efficient on its own, but they have trouble keeping up with one another when they are connected together."
|
|
Diehl said the group suspects that other classes of motor molecules, which are somewhat weaker than kinesin, may function better in groups. The team is carrying out follow-up experiments to see if that's the case, and they are examining how such distinctions may play a role in regulating cargo movement in cells.
|
|
Diehl's research group, which is located in Rice's new BioScience Research Collaborative, has spent years refining the tools used in the new study, and the work is paying off in numerous ways. Within the past four months, the group won an R01 grant from the National Institutes of Health worth more than $1.4 million, and Diehl also published a theoretical study of motor proteins with Rice chemist Anatoly Kolomeisky.
|

