| Nov 22, 2010 |
Success in developing groundbreaking electrolyte materials for solid oxide fuel cells
|
|
(Nanowerk News) SOFCs are environmental-friendly and efficient energy production devices. Reducing the operating temperature of SOFCs below 700°C is needed for a wide practical application of these devices. Yttrium-doped barium zirconate (BZY) is now considered as an alternative to the oxygen-ion conductor electrolytes conventionally used in SOFCs due to its higher bulk proton conductivity at low temperatures. BZY has not been exploited until now despite its excellent chemical stability because, when prepared as a ceramic polycrystalline material, it suffers from difficult sintering and proton conductivity is decreased by grain boundaries, which have a blocking effect.
|
|
The Fuel Cell Nano-Materials Group at the International Center for Materials Nanoarchitectonics in Japan has successfully developed two types of novel materials which satisfy all the three requirements for electrolyte: ion conductivity, chemical stability and sinterability, at high levels.
|
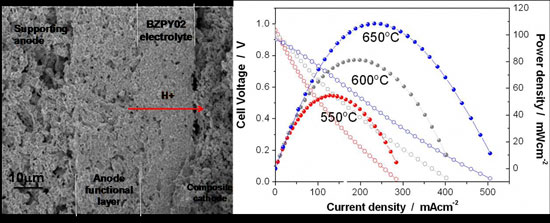 |
| Left: Cross section of BZPY-based single cell after fuel-cell testing. Four layers are clearly visible: from left, the supporting anode, the anode functional layer (about 22 µm thick), the electrolyte (about 20µm thick), and the composite cathode. Right: Voltage and power density curves of BZPY-based fuel cells measured at 550, 600 and 650°C in humidified hydrogen-+ambient air fuel cell experiments.
|
|
One is yttrium-doped barium zirconate with 10 mol% of praseodymium (BZPY). The addition of Pr improves the sinterability of BZY and dense samples are obtained after sintering at 1500°C for 8 hours. This material showed very high proton conductivity (above 0.01S/cm at 600°C), comparable to the proton conductivity of BCZY, now proposed for proton conductor electrolyte, but with significantly better chemical stability, thereby resulting in realistic applicability in fuel cell devices.
|
|
The other material is indium-doped barium zirconate (BZI) on a NiO-BZY anode substrate. During sintering at 1450°C, a dense electrolyte film is formed and simultaneously indium evaporates, being substituted by yttrium. The final result is the achievement of a dense BZY electrolyte film on a NiO-BZY anode, which cannot be obtained at the same temperature with direct processing. The fuel cells using this electrolyte film showed the largest fuel cell performance, 0.169 W/cm2 at 600°C, ever reported for BZY-based electrolytes. The BZY film made by this method shows excellent chemical stability, indicating its potential for long-term operation.
|
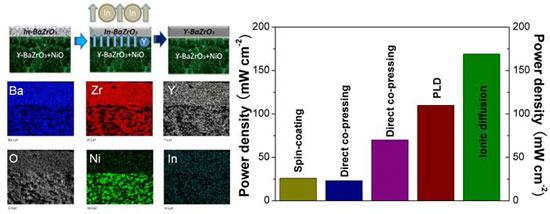 |
| Upper left: Schematic diagram of the procedure for preparing an anode supported BZY electrolyte film. Lower left: Elemental mapping, confirming the In3+ ions evaporation and Y3+ ions migration from the anode to the electrolyte layer, to occupy the In3+ sites. Right: Fuel cell performance of electrode-supported cells using BZY electrolytes prepared by different methods and measured at 600oC in the literature reports (spin-coating cell was tested at 800oC) as well as in the present study, indicating that the BZY cell in the present study shows the largest power density
|
|
These two materials are promising electrolyte materials for SOFC operating in the intermediate temperature range, 500 to 650°C, which allows reducing SOFC fabrication and operation costs, and thus accelerating their commercialization. The previous work of the group, published in Nature Materials on September 20th, introduced high-performance materials that show very high proton conductivity at even lower temperatures, but it was fabricated by using a special technology called pulsed laser deposition. In these new studies, high-performance electrolyte materials were obtained by simple co-pressing and subsequent sintering in the air, which is suitable for mass-production. This indicates the aforementioned results could accelerate the commercialization of SOFCs.
|


