| Posted: May 24, 2007 |
Urchin-shaped nano-batteries |
|
(Nanowerk News) Tweaking a standard chemical method to make nanotubes has provided researchers with a structure that looks just like a miniature sea urchin. These structures, composed of vanadium oxide, roll up during the reaction to form hollow tubes. By varying the reaction researchers were able to ‘grow’ these tubes into spherical/radial structures which resemble the common sea urchin ("Atomic Layer Structure of Vanadium Oxide Nanotubes Grown on Nanourchin Structures").
|
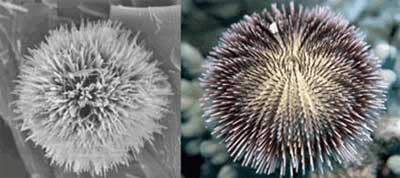 |
| Nano-urchin and real sea urchin (Image copyright: American Chemical Society)
|
|
"The nano-urchin’s spines could prove useful as scaffolding for further molecular construction, or as high volumetric density hosts for optically active nanoparticles", according to researchers at the Tyndall National Institute, part of University College Cork.
|
|
And what about applications! Colm O’Dwyer, a key researcher in this area outlined that on closer inspection, each nanotube in the urchin was almost identical to those seen during single nanotube synthesis, where flat sheets of vanadium oxide roll up into cylinders. These are the most uniform vanadium oxide tubes formed to date in terms of wall thickness, the number of layers, and the dimensions of the hollow centre. The unprecedented density of the urchin’s nanotubes provides a wealth of possible applications.
|
|
‘The high surface area could be used to support catalysis, for example’. But the researchers are particularly interested in using the structures as supports for other materials. Functionalization of the nano-urchin with organic surfactants would allow preferential uptake and attachment of choice molecules, nanoparticles or metallic ion-intercalation for charge storage applications.
|
|
Hoping that a battery based on nano-urchins could store the same amount of charge as a standard battery in a much smaller volume, the scientists have packed lithium atoms into the spaces between the tubes’ atoms. ‘Inserting the lithium into the urchin is not that difficult at all,’ said O’Dwyer, ‘The only difficult part, which we haven’t begun to do yet, is to sediment these on a polymer base, as for lithium polymer batteries.’
|
|
The work is the result of collaborative efforts between the Photonic Nanostructures group at Tyndall, the group of Prof. Guillermo Gonzalez at the Universidad de Chile, colleagues in Prof. E. Benavente’s group in the Universidad Tecnologica Metropolitana, Chile and Dr Simon Newcomb of Glebe Scientific, Co. Tipperary, Ireland. The research is supported by the SFI and the EU Network of Excellence PhOREMOST.
|

