| Mar 25, 2011 |
Nanotechnology points the way to greener pastures
|
|
(Nanowerk News) Nourishing crops with synthetic ammonia (NH3) fertilizers has increasingly pushed agricultural yields higher, but such productivity comes at a price. Over-application of this chemical can build up nitrate ion (NO3–) concentrations in the soil—a potential groundwater poison and food source for harmful algal blooms. Furthermore, industrial manufacturing of ammonia is an energy-intensive process that contributes significantly to atmospheric greenhouse gases.
|
|
A research team led by Miho Yamauchi and Masaki Takata from the RIKEN SPring-8 Center in Harima has now discovered an almost ideal way to detoxify the effects of ammonia fertilizers ("Highly Selective Ammonia Synthesis from Nitrate with Photocatalytically Generated Hydrogen on CuPd/TiO2"). By synthesizing photoactive bimetallic nanocatalysts that generate hydrogen gas from water using solar energy, the team can catalytically convert NO3– back into NH3 through an efficient route free from carbon dioxide emissions.
|
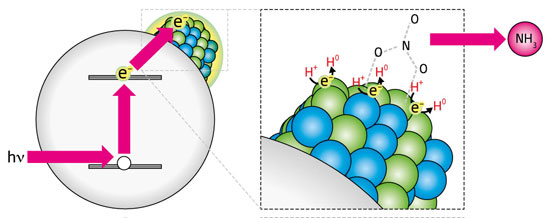 |
| Figure 1: Using ultraviolet radiation (hν) to produce energetic electrons (e-) enables a copper–palladium catalyst (green and blue spheres) to generate hydrogen (H0) without using fossil fuels. This material can then transform nitrate ions (NO3–) into ammonia (NH3).
|
|
Replacing the oxygen atoms of NO3– with hydrogen is a difficult chemical trick, but chemists can achieve this feat by using nanoparticles of copper–palladium (CuPd) alloys to immobilize nitrates at their surfaces and catalyzing a reduction reaction with dissolved hydrogen atoms. However, the atomic distribution at the 'nanoalloy' surface affects the outcome of this procedure: regions with large domains of Pd atoms tend to create nitrogen gas, while well-mixed alloys preferentially produce ammonia.
|
|
According to Yamauchi, the challenge in synthesizing homogenously mixed CuPd alloys is getting the timing right—the two metal ions transform into atomic states at different rates, causing phase separation. Yamauchi and her team used the powerful x-rays of the SPring-8 Center's synchrotron to characterize the atomic structure of CuPd synthesized with harsh or mild reagents. Their experiments revealed that a relatively strong reducing reagent called sodium borohydride gave alloys with near-perfect mixing down to nanoscale dimensions.
|
|
Most ammonia syntheses use hydrogen gas produced from fossil fuels, but the use of solar energy by the researchers avoids this. They found that depositing the nanoalloy onto photosensitive titanium dioxide (TiO2) yielded a material able to convert ultraviolet radiation into energetic electrons; in turn, these electrons stimulated hydrogen gas generation from a simple water/methanol solution (Fig. 1). When they added nitrate ions to this mixture, the CuPd/TiO2 catalyst converted nearly 80% into ammonia – a remarkable chemical selectivity that the researchers attribute to high concentrations of reactive hydrogen photocatalytically produced near the CuPd surface.
|
|
Yamauchi is confident that this approach can help reduce the ecological impact of many classical chemical hydrogenation reactions. "Considering the environmental problems we face, we have to switch from chemical synthesis using fossil-based hydrogen to other clean processes," she says.
|

