| Apr 18, 2011 |
Self-healing protection inspired by natural surfaces
|
|
(Nanowerk News) Clover leaves are superhydrophobic — water droplets sit on the surface of the leaf without wetting it due to a strong water-repelling action by the leaf surface. This superhydrophobicity is lost when the leaf suffers severe microscopic damage. Surprisingly however, even after such damage, clover leaves restore their anti-wetting properties in a matter of days by secreting wax-like compounds.
|
|
This natural self-healing property has inspired Zhou and colleagues from the Lanzhou Institute of Chemical Physics in China to develop a surface that can recover its water- and oil-repelling qualities after damage ("Self-healing superamphiphobicity").
|
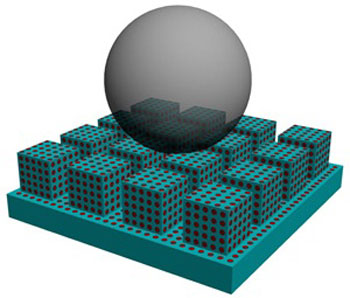 |
| Schematic diagram showing the structure of a self-healing superamphiphobic surface. (© 2011 RSC)
|
|
The team developed a self-healing surface that is both superhydrophobic and oil-repelling — a combination of properties known as superamphiphobicity that is not found in nature but which is very useful for industrial protective coatings. The surface itself is anodized alumina with a microscopically rough surface that is pitted with nanopores filled with perfluorooctanoic acid (see image). The perfluorooctanoic acid gives the surface its superamphiphobic properties, and the nanopores act as tiny reservoirs that release the liquid continuously to allow the surface's superamphiphobicity to be restored after damage.
|
|
To test the surface, the researchers repeatedly damaged it by exposure to oxygen plasma. They found that the surface was able to recover its superamphiphobicity up to six times. This self-healing ability effectively extends the functional life of the surface.
|
|
Zhou and his colleagues are now working to improve on these initial proof-of-concept findings. Through adjustments to the pore size, it is important to ensure that the perfluorooctanoic acid can migrate easily out onto the surface, but not so fast as to be wasted. "We are working with different materials, systematically changing the size of nanopores and trying to infiltrate the surface with more of the agent," says Zhou. "The lifetime of the surface could be extended even longer if we can hold more agent in the reservoir, and if the agent can migrate easily to the surface." By tuning these properties, the researchers expect their approach to lead to the development of new types of surface coatings, such as anti-icing and anti-corrosion surfaces, capable of maintaining their functionality for longer under very harsh conditions.
|

