| May 11, 2011 |
Metallic alloy 'snowflakes' enhance the efficiency and safety of rechargeable batteries
|
|
(Nanowerk News) The tiny porous frameworks of zinc–antimony (ZnSb) nanoflakes are set to have a big impact on future hybrid vehicles and pocket-sized electronic devices. Qingyu Yan, Bee Yen Tay and co-workers from the A*STAR Singapore Institute of Manufacturing Technology and Nanyang Technological University have deposited ZnSb nanostructures directly onto copper foil using a template-free electrochemical technique to produce a material that could enhance the charge-storage capacity and safety of lithium-ion batteries ("Template-Free Electrochemical Deposition of Interconnected ZnSb Nanoflakes for Li-Ion Battery Anodes").
|
|
Graphite is the anode of choice for most lithium batteries because it retains its structure quite well in the presence of lithium ions, giving the battery consistent charging behavior. Unfortunately, graphite has low charge-storage capacity, which limits the energy density of the battery. Pure lithium metal can also become intercalated into the graphite structure, which in extreme cases can cause the batteries to explode.
|
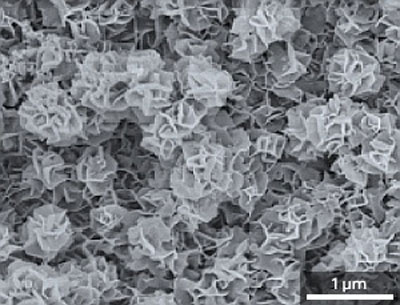 |
| Scanning electron microscopy image of the nanoflake zinc–antimony structure. (© 2011 ACS)
|
|
Incorporating materials with high theoretical charge-storage capacities, such as ZnSb, into the anodes of lithium-ion batteries could lead to thinner, lighter batteries that run at higher voltages, along with reduced lithium metal deposition. Unfortunately, antimony-based alloys can undergo destructive volume changes after repeated interactions with lithium ions, leading to early battery failure.
|
|
Yan, Tay and their co-workers overcame ZnSb's deformation problems by turning to the exotic world of nanotechnology. Through an 'overpotential' electrochemical deposition process that forces the rapid growth of crystals onto copper substrates, the team developed a method to produce ZnSb alloys containing honeycomb-like internal nanoscale pores, termed a 'nanoflake' structure, without the need for a template (see image). By altering parameters like deposition voltages and zinc–antimony precursor ratios, the team could also manipulate ZnSb crystals into distinct nanowire and nanoparticle shapes.
|
|
The researchers had a hunch that the enhanced surface area of the nanoflakes would help buffer any swings in volume due to lithium ion interactions. After coating the ZnSb nanostructures with carbon to improve durability, the team tested the new anode in a lithium-ion battery and found their suspicions to be correct—not only did the ZnSb nanoflake structure have a steady discharge capacity one-third higher than commercial batteries, they could also be recharged repeatedly without any structural changes. The intimate connection between the nanoflakes and the copper electrode also improved the battery's charge-carrying efficiency to a remarkable 98%.
|
|
"The fast, easy and cheap fabrication of ZnSb nanostructures without a template makes it possible to prepare anodes with improved electrochemical performance using large-scale electroplating processes," says Tay. "This system has the potential to form the basis for a new generation of lithium-ion batteries with higher energy densities."
|

