| May 30, 2011 |
Nanomedicine allows 'self-diagnosing' therapy with theragnostic drug nanocarriers
|
|
(Nanowerk News) Early diagnosis and accurate drug delivery are vital to effective cancer therapy. Jin Suck Suh from Yonsei University and colleagues from a number of institutes in Korea have now developed a new type of particle that can achieve both: diagnosing cancer cells and delivering targeted therapeutic agents ("pH-Triggered Drug-Releasing Magnetic Nanoparticles for Cancer Therapy Guided by Molecular Imaging by MRI").
|
|
The particles are called 'theragnostic' nanocarriers and consist of poly(ethylene glycol) surfactant molecules that self-organize into tiny capsules to encase anticancer druges like doxorubin (see image).
|
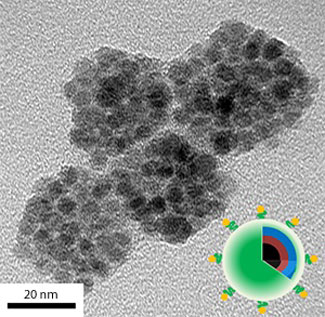 |
| A transmission electron microscopy image of pH-sensitive theragnostic nanocarriers loaded with doxorubin.
|
|
Rapidly growing cancers increase glucose metabolism in cells through two pathways, called aerobic glycolysis and lactic acid secretion, that produce an acidic microenvironment. Suh's team modified the surfactant with a pH-responsive functional group, which allowed the resulting nanocarriers to release over 80% of doxorubin to low-pH cells but only 30% of the drug under normal physiological conditions. Suh explains that, at neutral pH, the surfactant binds the encapsulated doxorubin through intermolecular forces, keeping the drug inside the capsules. Upon reaching the low-pH microenvironment of cancer cells, however, these intermolecular interactions are weakened and the doxorubin is released.
|
|
The researchers additionally decorated the nanocarriers with manganese ferrite — a contrast agent used for magnetic resonance imaging — and an antibody called 'anti-HER2/neu', which is used to treat breast cancer. The combination of contrast agent and antibodies caused the nanocarriers to produce strong MRI signals only in the presence of the HER2/neu receptor in breast cancer cells. Fluorescence imaging and MRI also revealed that the nanocarriers readily penetrated into the cell cytoplasm by binding to the receptor. Once in the cell, the doxorubin was released and accumulated near the nucleus.
|
|
In vivo MRI experiments showed that nanocarriers injected in mice with cancer accumulated in tumors within 24 hours, then slowly receded over 48 hours. After administering the doxorubin-loaded nanocarriers every three days for nine days, the researchers observed suppressed tumor growth, demonstrating the potency of the strategy. At the same time, the weights of the injected mice did not change — proof that harmful side effects were minimal.
|
|
The team is further investigating cancer metabolism regulation in order to generate other smart nanosystems that integrate different anticancer therapeutic agents and imaging tools. "We plan to develop multimodal imaging tools to achieve accurate cancer diagnosis and therapy," says Suh.
|

