| Jun 01, 2011 |
New inorganic semiconductor layers hold promise for solar energy
|
|
(Nanowerk News) A team of researchers from the University of Chicago and the U.S. Department of Energy's (DOE) Argonne National Laboratory has demonstrated a method that could produce cheaper semiconductor layers for solar cells ("Band-like transport, high electron mobility and high photoconductivity in all-inorganic nanocrystal arrays").
|
|
The inorganic nanocrystal arrays, created by spraying a new type of colloidal "ink", have excellent electron mobility and could be a step towards addressing fundamental problems with current solar technology.
|
|
"With today's solar technology, if you want to get significant amounts of electricity, you'd have to build huge installations over many square miles," said team leader Dmitri Talapin, who holds a joint appointment with Argonne and the university. But because current solar cells are based on silicon, which is costly and environmentally unfriendly to manufacture, they aren't cost-effective over large areas. The challenge for scientists is to find a way to manufacture large numbers of solar cells that are both efficient and cheap.
|
|
One possibility to make solar cells more economically would be to "print" them, similar to how newspapers are printed. "You'd use a kind of 'ink,' stamped on using a roll technology with a flexible substrate," Talapin said.
|
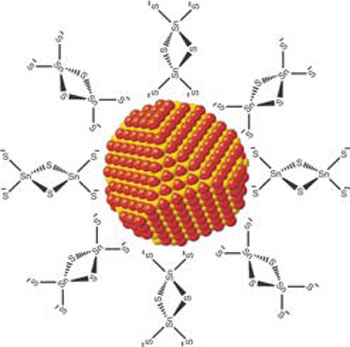 |
| Inorganic surface ligands enable facile electron transport between quantum dots and opened novel opportunities for using nanostructures in solar cells.
|
|
Solar cells have several layers of different materials stacked on top of each other. The team focused on the most important layer, which captures sunlight and converts it into electricity. This layer, made of a semiconducting material, must be able to transform light into negative and positive electrical charges but also easily release them to move further along the material to generate electrical current.
|
|
Many methods to grow the semiconductors need high temperatures, but a cheaper approach would be to make them in solution. This, however, requires a precursor that is soluble.
|
|
The team developed that precursor using quantum dots. Small grains of semiconductors, suspended in a liquid, are "glued" together with new molecules called "molecular metal chalcogenide complexes." The process heats the material to about 200 degrees Celsius, much lower than the temperatures required for manufacturing silicon solar cells. The result is a layer of material with good semiconducting properties.
|
|
"The electron mobility for this material is an order of magnitude higher than previously reported for any solution-based method," Talapin said.
|
|
The team used intense X-rays from the DOE Office of Science's Advanced Photon Source at Argonne to watch as the semiconductor film was created.
|
|
"We believe that we could make very competitive solar cells with these nanoparticles," Talapin said.
|
|
Talapin said the success played on the complementary partnership between the University of Chicago and Argonne's Center for Nanoscale Materials. "At the university we have great students and postdocs who can do a lot of the theoretical chemistry, which requires a lot of manpower," Talapin said, "but Argonne is a fantastic place to do research that requires sophisticated instrumentation and infrastructure."
|
|
The research was supported by the Office of Naval Research and a National Science Foundation CAREER award. Work at the Center for Nanoscale Materials and the Advanced Photon Source was supported by the DOE's Office of Science.
|

