| Jun 03, 2011 |
Being stressed without ripping - flower-like defects in graphene
|
|
(Nanowerk News) Beyond its ability to conduct electrons almost without resistance, the nanomaterial graphene also has amazing mechanical properties, including high strength that could one day make it useful in lightweight, robust structures. But this material is not without flaws – including a family of flower-like defects that could detract from its electronic and mechanical properties.
|
|
In a paper published in the journal Physical Review B, researchers at the Georgia Institute of Technology and the National Institute of Standards and Technology (NIST) have described a family of seven potential defect structures that may appear in sheets of graphene and imaged examples of the lowest-energy defect in the family.
|
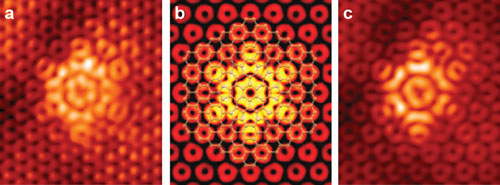 |
| Flower-like defects in graphene are shown in these images (a and c) produced by a scanning tunneling microscope. Image b was created by a computer model. (Images: Eric Cockayne, NIST)
|
|
The defects may arise to help relieve mechanical stress in graphene's carbon-atom honeycomb structure by allowing atoms to spread out and occupy slightly more space. Such stress may arise during the growth of graphene or by stretching the graphene sheet.
|
|
"For an engineer interested in the mechanical properties of graphene to create atom-thick membranes, for instance, it would be very important to understand these kinds of properties, which could give rise to plastic deformation of the material," said Phillip First, one of the paper's co-authors and a professor in the Georgia Tech School of Physics. "For instance, it may be that these defects are just one part of the kinetic pathway to failure for a strained sheet of graphene."
|
|
For electronic applications, the defects could deflect electrons and cause backscattering that would increase the resistance of the material – like a rock in a stream slows the flow of water. However, First says improved growth techniques developed since the defect study began may eliminate that concern.
|
|
"With the growth techniques that have now been developed using silicon carbide, we typically do not see these defects," he noted. "The defects occur on material that we know to be of a lower quality because of the growth conditions or substrate preparation."
|
|
Defects can appear due to the movement of carbon atoms at high temperatures, explained NIST Fellow Joseph Stroscio. Rearrangements of graphene that require the least amount of energy involve switching from the standard six-member carbon rings to structures containing either five or seven atoms. The NIST researchers have discovered that stringing five- and seven-member rings together in closed loops creates a new type of defect or grain boundary loop in the honeycomb lattice.
|
|
According to NIST researcher Eric Cockayne, the fabrication process plays a big role in creating the defects.
|
|
"As the graphene forms under high heat, sections of the lattice can come loose and rotate," he said. "As the graphene cools, these rotated sections link back up with the lattice, but in an irregular way. It's almost as if patches of the graphene were cut out with scissors, turned clockwise, and made to fit back into the same place. Only it really doesn't fit, which is why we get these flowers."
|
|
So far, only the flower defect, which is composed of six pairs of five- and seven-atom rings, has been observed. Modeling of graphene's atomic structure by the NIST team suggests there might be a veritable bouquet of flower-like configurations. These configurations – seven in all – would each possess its own unique mechanical and electrical properties, Cockayne said.
|
|
First hopes the team can continue studying the defects, both to learn whether their formation can be controlled and to clarify the role of defects in the material's mechanical properties.
|
|
"Graphene is strong and light, so the mechanical properties are of great interest," he noted. "Understanding just how it rips apart is an interesting question that has important implications. But even with these defects, graphene is still spectacularly strong."
|

