| Jul 25, 2011 |
Carbon nanotubes: Charge with a twist
|
|
(Nanowerk News) Single-walled carbon nanotubes (SWCNTs) are poised to revolutionize the engineering of high-performance electronic devices. These rolled-up carbon sheets behave like metals or semiconductors according to their orientation and degree of twisting, or chirality. Unfortunately, the mechanisms that govern the formation of these cylindrical structures remain unclear, making their geometry difficult to control during synthesis. Now, using quantum simulations, a team led by Yuan Chen from Nanyang Technological University in Singapore has elucidated the role of charge transfer in chiral nanotube growth ("Charge Transfer between Metal Clusters and Growing Carbon Structures in Chirality-Controlled Single-Walled Carbon Nanotube Growth").
|
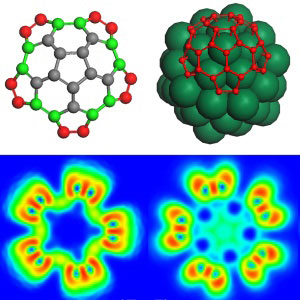 |
| Plots showing the charge distribution in edge atoms of chiral carbon nanotube 'caps' before (left) and after (right) binding to the metal cluster (green). (© 2011 ACS)
|
|
Many theoretical studies have been made to investigate the influence of energies, structures and growth kinetics in the formation of SWCNTs. However, these studies have been unable to provide a convincing explanation of the chirality selection observed experimentally. "Our initial intention was to find out how the adhesion energies and chemical potentials of growing carbon structures on metal nanoparticles influence chirality selection in SWCNT growth," explains Chen.
|
|
His team carried out calculations for various nanotube structures growing on a nickel cluster. Unfortunately, these simulations revealed very little in terms of energy differences between the investigated compounds, suggesting that the cluster did not drive chirality — a major discrepancy from experimental results. Conversely, the researchers noticed that, in agreement with transmission electron microscopy observations, the geometries of the cluster and growing structures deformed upon binding to one another, indicative of a highly reactive interface between the cluster surface and the extremities of the growing nanotube.
|
|
Chen says that it is the importance of charge transfer in explaining and predicting structural change and reactivity in organometallic chemistry that prompted his team to investigate the impact of this phenomenon on chiral SWCNT growth.
|
|
A detailed analysis of electron densities revealed that the metal catalyst plays a central role in activating the edge carbon atoms. Specifically, the researchers found that when carbon structures adhere to a cluster, negative charges move from the metal to the carbon atoms (see image). Moreover, this charge redistribution, which occurs exclusively at the cluster–carbon structure boundary, displays specific patterns depending on the carbon 'cap' chirality. "It enhances the reactivity of carbon structure edges, resulting in reaction sites with different reactivity," says Chen.
|

