| Sep 18, 2011 |
Electrical energy storage from -50 C to 100 C
|
|
(Nanowerk News) A team of transatlantic researchers from three countries report the development of a novel system that may help to store and use electrical energy from extremely low to very hot temperatures. The paper was published in the Journal of Physical Chemistry Letters ("Capacitive Energy Storage from -50 to 100°C Using an Ionic Liquid Electrolyte").
|
|
Most batteries are limited in their ability to operate at very low or very high temperatures. While performance of electrochemical capacitors (also referred to as supercapacitors or ultracapacitors) is less dependent on the temperature, present-day devices still cannot cover the entire range needed for automotive and electronics applications under a variety of environmental conditions. Now, thanks to an ongoing collaboration between scientists at Drexel University (Philadelphia, PA) and Paul Sabatier University (Toulouse, France), these energy storage devices are reported to work in an impressive range of temperatures, from -50 to over 100°C. The feat was achieved through the combination of advanced materials called ionic liquids and nanostructured carbons.
|
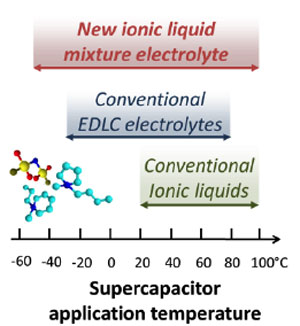 |
|
Ionic liquids are unique salts that, unlike table salt, remain in liquid state at room temperature. This in turn allows their use as electrolytes in supercapacitors and batteries. They are not flammable, providing a safer technology. While ionic liquids have attracted much interest for electrochemical applications, the recent work of Prof. Patrice Simon's group and his student Rongying Lin, in collaboration with industrial partners SOLVONICS, is the first to show that by employing a mixture of them it is possible to produce an energy storage device that can be used from the Sahara to the Arctic. When two specially selected ionic liquids are mixed in a certain proportion, the mixture does not freeze at temperatures when individual liquids would already solidify. This can dramatically extend the temperature range of electrical energy storage, thus defying the conventional wisdom that ionic liquids can only be used as electrolytes above room temperature.
|
|
Nanostructured carbons are the other component that is critical for the device's performance. Ionic liquid electrolyte will not work with a conventional activated charcoal used in commercial supercapacitors. Small particles with a large external surface facilitate ion transport as ions no longer need to be squeezed through a network of narrow pores. The first material studied by the research team was a thin film of vertically aligned carbon nanotubes, which was developed by an industrial collaborator AIXTRON employing an aluminum foil, like the one used in every household, as the substrate. The second carbon nanomaterial, carbon onions, was obtained from vacuum annealing yet another carbon nanostructure: nanodiamond, which is approximately one million times smaller than a one-karat diamond solitaire. From this annealing process, multi-shelled carbon spheres are derived with concentric graphitic shells resembling layers in an onion. This material was recently reported to yield extremely fast charge/discharge rate dramatically cutting down the time required to fully charge a supercapacitor.
|
|
Dr. Yury Gogotsi, Trustee Chair Professor of Materials Science and Engineering at Drexel University, Director of the A.J. Drexel Nanotechnology Institute, and a co-author of the paper states: "Extending the temperature range for batteries or supercapacitors is of great importance since this could solve the problem of the operation in severe conditions required for automotive, aerospace, and power electronics applications. Anyone who tries to start a car on a cold winter morning, cranking the ignition several times, knows that car batteries do not work well at low temperatures. These devices can be used for starting a truck or locomotive engines in Alaskan winter, but will not catch fire in Arizona desert."
|
|
"This scientific discovery may have important implications for the quickly growing area of renewable energy, where electricity storage is the bottleneck for further growth and wider use of wind and solar," adds Dr. Volker Presser. "Ionic liquid based supercapacitors can be exposed to varying weather conditions and their cycle life is about 100 times longer than that of present day batteries." Another important application is that hybrid and electric vehicles may be able to use this technology for regenerative breaking, creating cleaner ways to power a 21st century lifestyle. The new ionic liquid supercapacitors can make a huge difference – also, because they are non-flammable and non-volatile making electric cars much safer than any combustion engine.
|
|
The leader of the French team Prof. Patrice Simon believes that "these results show that a proper design of the carbon electrode structure in conjunction with an electrolyte formulation offers an opportunity for expanding the operation range of electrical energy storage devices and adapting them for applications under extreme climatic conditions" and he continues: "The now-obtained temperature range for our new supercapacitors is remarkable and this is certainly not the limit."
|

