| Jan 25, 2006 |
Development of nanoparticle libraries for biosensing
|
|
(Nanowerk News) Over the past couple of years, researchers have developed a number of standardized techniques for attaching an antibody or protein to the surface of a nanoparticle in order to create a targeted drug delivery vehicle or imaging agent. This approach works well when trying to target a known cancer biomarker, but the fact is that today, researchers have only a few such markers to choose from, and many types of cancers do not express those few known markers.
|
|
In an attempt to overcome this limitation, a team of researchers at the Massachusetts General Hospital has taken a different approach, creating a large library of nanoparticles, each with a different small molecule decorating its surface. They then screen this library to see if any of the nanoparticles will bind to any number of cancer cells while ignoring healthy cells.
|
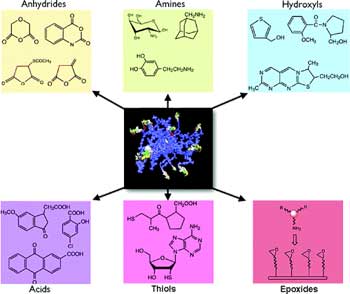 |
Nanoparticle library. Examples of small molecules attached to amino-CLIO to create a 96-member library (to fill one microtiter plate) that was subsequently used (Source and Copyright: American Chemical Society) |
|
Reporting its work in the Dec. 30, 2005 online issue of Bioconjugate Chemistry, a team led by Ralph Weissleder, M.D., and Lee Josephson, Ph.D., describes the methods they use for attaching a variety of small organic molecules to the surface of a magnetic and fluorescent nanoparticle. The researchers chose to use small molecules of "nonbiological origin" with an eye on keeping costs and regulatory burdens low should any of these nanoparticles prove clinically useful.
|
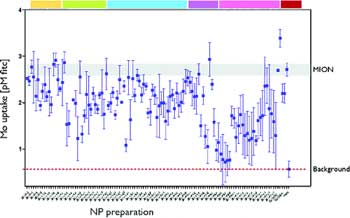 |
Screening of nanoparticle library in macrophages. Small molecule modified nanoparticle preparations were incubated with U937 macrophages, and cellular uptake was determined by a FITC-mediated immuno ELISA assay.13 Note the variability in uptake upon small molecule modification compared to base material (MION). (Source and Copyright: American Chemical Society) |
|
The researchers also worked out a method to ensure that each chemical preparation went as planned. This latter step is critically important in order to distinguish between modified nanoparticles that have no biological activity and those that have no activity because the expected chemical modifications never occurred in the first place. Finally, in order to automate the screening process, the researchers also developed two techniques for “printing” the resulting libraries of modified nanoparticles onto glass slides or for adding each member of the library to the tiny indentations on a standard 96-well assay plate used in a wide variety of screening technologies.
|
|
In a demonstration experiment, the investigators prepared a 96-well plate in which each well contained macrophages, a type of immune system cell that has a propensity to engulf nanoparticles. They then added individual members of the library to each well and identified modified nanoparticles that were not taken very effectively by macrophages (see illustration). The macrophage-avoiding nanoparticles may be able to more effectively deliver drugs to tumors since more of them may be able to reach their target rather than be eliminated from the body by macrophages.
|
| The full article can be downloaded here (PDF download 150 KB).
|


