| Jan 07, 2012 |
Novel microinjection technology delivers nanoparticles to the back of the eye
|
|
(Nanowerk News) Technology developed by researchers at the Georgia Institute of Technology and Emory University for delivering drugs and other therapeutics to specific locations in the eye provides the foundation for a startup company that has received a $4 million venture capital investment.
|
|
The Atlanta-based startup, Clearside Biomedical, plans to develop microinjection technology that will use hollow microneedles to precisely target therapeutics within the eye. If the technique proves successful in clinical trials and wins regulatory approval, it could provide an improved method for treating diseases that affect the back of the eye, including age-related macular degeneration.
|
|
The technology was developed in collaboration between the research groups of Mark Prausnitz, a Regents' professor in Georgia Tech's School of Chemical and Biomolecular Engineering, and Henry Edelhauser, a professor in the Department of Ophthalmology at Emory School of Medicine. Research leading to development of the technology was sponsored by the National Institutes of Health (NIH).
|
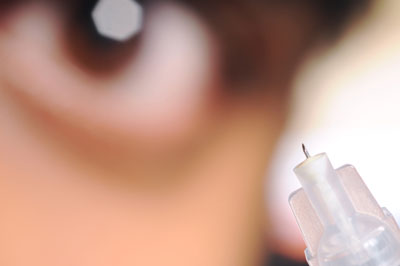 |
| Postdoctoral fellow Samirkumar Patel displays a prototype microneedle used to inject therapeutics into specific locations in the eye. The technology could allow doctors to target drugs to locations in the eye that are now difficult to reach.
|
|
"We expect that targeting drug delivery within the eye will be helpful because we should be able to concentrate drugs at the disease sites where they need to act, and keep them away from other locations," said Prausnitz. "This could reduce side effects and possibly also decrease the dose required."
|
|
Prior to this development, drugs could be delivered to the retinal tissues at the back of the eye in three indirect ways: (1) injection by hypodermic needle into the eye's vitreous humor, the gelatinous material that fills the eyeball, (2) eye drops, which are limited in their ability to reach the back of the eye, and (3) pills taken by mouth that expose the whole body to the drug.
|
|
The technology developed by Georgia Tech and Emory uses a hollow micron-scale needle to inject therapeutics into the suprachoroidal space located between the outer surface of the eye -- known as the sclera -- and the choroid -- a deeper layer that provides nutrients to the rest of the eye. Preclinical research has demonstrated that fluid can flow between the two layers, where it can spread out to the entire eye, including structures such as the retina that are now difficult to reach.
|
|
By targeting this suprachoroidal space using microscopic needles, the researchers believe they can reduce trauma to the eye, make drugs more effective and reduce complications. The new delivery method could help advance a new series of drugs being developed to target the retina, choroid and other structures in the back of the eye.
|
|
"This is a significant advance in the field of ophthalmology," said Edelhauser. "Until now, it has been difficult to target drug delivery to specific locations within the eye. This new microneedle technology enables precise drug targeting to the suprachoroidal space and other locations within the eye."
|
|
In research reported in the January 2011 issue of the journal Pharmaceutical Research ("Suprachoroidal drug delivery to the back of the eye using hollow microneedles"), the Georgia Tech-Emory team demonstrated for the first time that this technique can be used to deliver nanoparticles and microparticles to specific parts of the eye. In later research, they also showed that microneedle injections into the suprachoroidal space rapidly resulted in concentrations of drugs and particles that could be maintained for several months.
|
|
Between two and three million eye injections are made each year, many of them to treat age-related macular degeneration (AMD). The researchers believe that the microneedle-based technique could be useful for treating both AMD and glaucoma, as well as other ocular conditions related to diabetes.
|
|
The $4 million in funding for Clearside Biomedical will come from Hatteras Venture Partners, a venture capital firm based in Research Triangle Park, N.C. Hatteras focuses on seed and early-stage investments in companies developing products in biopharmaceutical, medical device, diagnostic and related human health areas.
|
|
"Clearside Biomedical represents an ideal fit for Hatteras Discovery as the platform technology is highly innovative, based on elegant science and the lead product is expected to be in clinical trials in the United States in less than 18 months," said Christy Shaffer, Ph.D., venture partner and managing director of the Hatteras Discovery Fund.
|
|
So far, the technique has been tested only in animals. The Hatteras funding will allow the company to conduct additional efficacy and safety testing needed to seek regulatory approval. The company's first product is expected to address macular edema and retinal vein occlusion.
|
|
Clearside was formed with the assistance of Georgia Tech's VentureLab program, which helped obtain early-stage seed funding from the Georgia Research Alliance. Georgia Tech VentureLab also helped the founders connect with the company's president and CEO, Daniel White, a veteran ophthalmic entrepreneur. Before joining Clearside, White was a co-founder of Alimera Sciences, an Atlanta company that is developing ophthalmic pharmaceuticals.
|
|
Two researchers from the Prausnitz lab who have been involved in development of the ocular drug delivery technique will also join the company. They are Samirkumar Patel, a postdoctoral researcher and Vladimir Zarnitsyn, a research scientist.
|

