| May 22, 2012 |
Scientists find gold-plated fossil solution
|
|
(Nanowerk News) Microscopic fossils, by their nature, contain microscopic detail – as do some macrofossils if they are extremely well preserved. To see that detail under a scanning electron microscope, the standard technique is to coat the fossil with a conductive metal such as silver, palladium or gold, which ensures that the surface of the specimen conducts evenly and no charge builds up. Gold-coating is also used in optical microscopy to provide a reflective surface for transparent or translucent specimens.
|
|
The problem is: how do you remove the gold afterwards so that you can further study the specimen itself? The gold has to react with something, but it is a very inert metal (one of the reasons why it is so useful) so you need either a very powerful oxidising agent, which is potentially liable to damage your specimen, or something which will stabilise the gold ions so that a mild oxidising agent can be employed. Which sounds a better idea, except that the standard procedure is to stabilise the gold using cyanide, with all the dangers and problems inherent in that infamously unpleasant chemical.
|
|
Here at Leicester, our chemists have been making great strides in the use of ionic liquids as solvents; that is, salts which are liquid at room temperature. These ionic liquids are safe to handle, easy to store and environmentally friendly, and much of our Ionic Liquids Group's research has been into industrial processes such as metal-plating. But now Dr Gero Frish and PhD student Jennifer Hartley from our Department of Chemistry have teamed up with Professor Mark Purnell and research associates Laurent Darras and David Jones* from our Department of Geology to apply similar techniques to fossil microscopy. The results of their research have been published in the online journal Palaeontologia Electronica ("Non-destructive, safe removal of conductive metal coatings from fossils: a new solution").
|
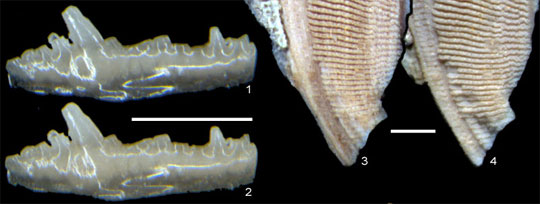 |
| Spot the difference: Optical micrographs before gold coating (1, 3) and after removal of same (2, 4). On the left are teeth from Wurmiella excavate, an eel-like chordate called a conodont; on the right is a scale from a lobe-finned fish (probably Onychodus).
|
|
The Leicester team used iodine as an oxidising agent, dissolved in a chemical called Ethaline which is a 'deep eutectic solvent', ie. a mixture of two chemicals that has a melting point much lower than either of its ingredients. Ethaline is produced by mixing choline chloride with ethylene glycol at about 60°C. It can easily be made in large quantities and stored at room temperature, and it dissolves iodine nearly 700 times better than water: at 60°C, a kilogram of Ethaline will happily dissolve 200g of iodine – and the resulting solution is stable for a week.
|
|
A range of specimens from the Department of Geology's fossil collections were used, including calcareous fossils of plankton and phosphatic fossils of lungfish scales and mineralised muscle fibres. Micrographs taken before the application of a 30nm layer of gold, and after its removal, show how effective the process is. But it's not just efficient, it's also very cheap, very quick (less than 12 hours to fully remove the gold) and very safe. The cyanide technique requires a fume cupboard whereas the ionic liquid process can be done on a workbench without even opening a window.
|
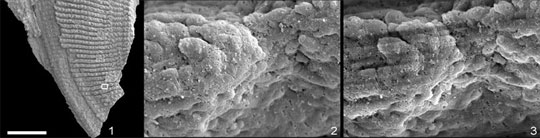 |
| Before and after. This SEM image of a tiny part of the Onychodus scale (indicated on the left) clearly shows how well-preserved the detail is after the removal of gold-coating using an Ethaline-iodine solution.
|
|
Finally the specimens are given a quick rinse in dilute potassium iodide and then deionised water. The solution contains so much iodine that it can be used several times – after which it can be simply tipped down the drain without any environmental concerns.
|
|
On top of all these advantages, the Leicester technique won't affect the adhesive used to attach the specimen to the microscope slide (provided it isn't water-soluble), which makes handling so much easier.
|
|
In their paper, the team point out that the wide range of ionic liquids available means that bespoke solutions can be easily determined for other types of fossil and/or metallic coatings. But they do recommend a 'trial run' of any new solvent before applying it to any particularly rare or valuable specimen, just in case…
|


