| May 31, 2012 |
Scientists hone in on size and environmental influence of the quantum dots used in hybrid solar cells
|
|
(Nanowerk News) Sometimes to answer big questions, you need to start small-very small. Scientists from Pacific Northwest National Laboratory's Chemical Imaging Initiative did just that when they analyzed cadmium selenide, or CdSe, quantum dots. Quantum dots are nanometer-sized particles that have different optical and electronic properties than their bulk materials. The team showed how size and environment unexpectedly alter the dots' structure (see paper in Physical Chemistry Letters: "Probing the Size- and Environment-Induced Phase Transformation in CdSe Quantum Dots").
|
|
Understanding the chemistry involved in these tiny transformations has applications in hybrid solar cells, where improving the electron mobility can ultimately enhance their overall efficiency and ability to contribute to the nation's energy needs.
|
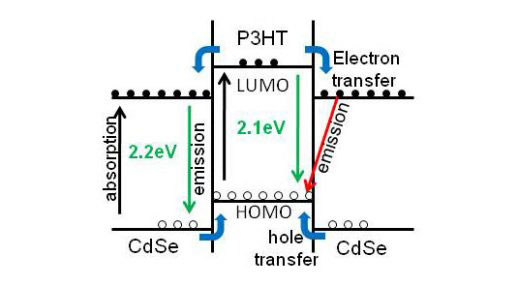 |
| The proposed charge transfer mechanism
|
|
Why it matters: The majority of quantum dot studies focus on improving the charge transport and collection and the efficiency of the solar cells, but few focus on the underlying chemical mechanics. This study was the first to examine how the surrounding environment and size chemically induce changes in the structure of semiconductor quantum dots. Ultimately, elucidating the chemical and the electronic structure interactions of CdSe quantum dots will illustrate mechanisms that will advance the hybrid solar cell technologies.
|
|
"Because hybrid solar cells have great potential in commercial applications, most folks start by looking at the overall cell efficiency, and the fundamental understanding of the chemical and electronic structure interactions is being overlooked," said Dr. Ajay Karakoti, a PNNL scientist and the study's lead author. "We are trying to understand the fundamental interactions. We want to make sure the chemical and structural integrity do not change. In this case, it did. That was unexpected."
|
|
Understanding the chemistry involved in these tiny transformations has applications in hybrid solar cells, where improving the electron mobility can ultimately enhance their overall efficiency and ability to contribute to the nation's energy needs.
|
|
Methods: Various imaging, spectroscopy, and diffraction instruments at EMSL were used to carry out this work. The instruments included micro X-ray diffraction, X-ray photoelectron spectroscopy, and ultraviolet-visible absorption and emission spectroscopy. Karakoti and co-author Dr. Ponnusamy Nachimuthu were quick to explain that the EMSL user facility simplified access to the diverse instrumentation and personnel skill sets necessary for their research. Combining the spectroscopy with imaging provided the chemical signature along with the spatial distribution of the elements.
|
|
What's Next? While they initially conducted their study using CdSe quantum dots in their native environment and drop-cast on a silicon wafer, this was a small step toward more detailed examinations of quantum dots incorporated in a polymer matrix. Building on this research, the team has expanded its focus in determining the source of defect states in CdSe quantum dots with decreasing size and its role in phase transformation, the electronic structures, and the band alignments.
|

