| Posted: September 10, 2008 |
Mysterious nature of glass explained by new theory |
|
(Nanowerk News) Archaeological evidence suggests that glass was first made in the Middle East sometime around 3000 B.C. However, almost 5,000 years later, scientists are still perplexed about how glassy materials make the transition from a molten state to a solid. Richard Wool, professor of chemical engineering at UD, thinks he has the answer.
|
|
In a paper to be published later this year in the Journal of Polymer Science Part B: Polymer Physics, Wool documents a new conceptual approach, known as the Twinkling Fractal Theory (TFT), to understanding the nature and structure of the glass transition in amorphous materials. The theory provides a quantitative way of describing a phenomenon that was previously explained from a strictly empirical perspective.
|
|
“The TFT enables a number of predictions of universal behavior to be made about glassy materials of all sorts, including polymers, metals and ceramics,” Wool says.
|
|
What distinguishes glasses from other materials is that even after hardening, they retain the molecular disorder of a liquid. In contrast, other liquids--for example, water--assume an ordered crystal pattern when they harden. Glass does not undergo such a neat phase transition; rather, the molecules simply slow down gradually until they are stuck in an odd state somewhere between a liquid and a solid.
|
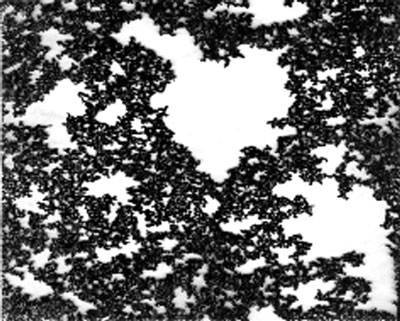 |
| As a liquid cools toward the glassy state, the atoms form clusters that eventually become stable and percolate near the glass transition temperature. The percolating clusters are stable fractals, or structures with irregular or fragmented shapes. (Image: Richard Wool)
|
|
Another difference between glasses and more conventional materials is that their transition from the liquid to the solid state does not occur at a standard temperature, like that of water to ice, but instead is rate-dependent: the more rapid the cooling, the higher the glass transition temperature.
|
|
Wool discovered that as a liquid cools toward the glassy state, the atoms form clusters that eventually become stable and percolate near the glass transition temperature. The percolating clusters are stable fractals, or structures with irregular or fragmented shapes.
|
|
“At the glass transition temperature, these fractals appear to twinkle in a specific frequency spectrum,” Wool says. “The twinkling frequencies determine the kinetics of the glass transition temperature and the dynamics of the glassy state.”
|
|
The theory has been validated by experimental results reported by Nathan Israeloff, a physics professor at Northeastern University. “He was not aware of the TFT,” Wool says, “but his results fit my theory in extraordinarily explicit detail.”
|
|
TFT was developed as an outgrowth of Wool's research on bio-based materials such as soy-based composites. “It was my need to solve issues in the development of these materials that led me to the theory,” he says.
|
|
In a recent article in the Science Times section of The New York Times about the nature of glass, journalist Kenneth Chang refers to the puzzle of the glass transition as “an arguably Nobel-worthy problem.” While Wool acknowledges that it is an important breakthrough, he says that it will take time for the impact to be clear. “It will have to play itself out in the scientific literature,” he says.
|
|
For now, Wool is content to view the theory as a portal into materials science and solid-state physics that others can use to go in new directions. “Acceptance will come when people recognize that it works,” he says.
|
|
TFT has the potential to contribute to better understanding of such phenomena as fracture, aggregation and physical aging of materials. “It is also giving us new insights into the peculiarities of nanomaterials, which behave very differently from their macroscopic counterparts,” Wool says.
|
|
Wool, who earned his doctorate at the University of Utah, joined the UD faculty in 1995. An affiliated faculty member in the Center for Composite Materials, he was recently featured on the Sundance Channel series “Big Ideas for a Small Planet.”
|

