| Posted: September 26, 2008 |
Study obtains protein structures more efficiently using a combination of techniques |
|
(Nanowerk News) The chances of obtaining crystals of sufficient quality and quantity to allow determination of three-dimensional protein structures using synchrotron radiation are significantly increased using a mix of robots geared to different crystallization techniques. That is the conclusion of a screening study by researchers in Japan, led by Seiki Kuramistu of RIKEN’s SPring-8 Center in Harima, recently reported in Acta Crystallographica ("Crystallization screening test for the whole-cell project on Thermus thermophilus HB8").
|
|
The work was part of the whole-cell project on the bacterium Thermus thermophilus HB8, which is found naturally in hot springs at temperatures of up to 85°C. The aim of the project is to increase understanding of cells at a molecular level by determining the structures and functions of all proteins encoded by genes. Thermus was chosen as a model organism because it has a minimal set of genes which codes for about 2,000 proteins which are highly stable for analysis and more than 70% of which have human equivalents.
|
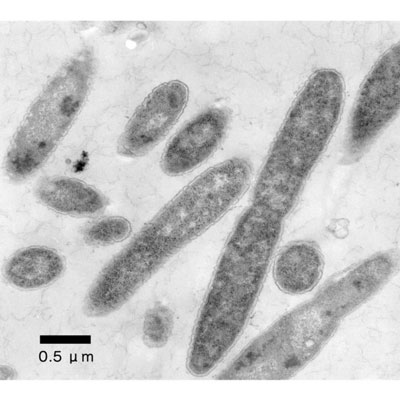 |
| An electron micrograph of Thermus thermophilus HB8. (Image: RIKEN)
|
|
The standard means of determining protein structure, x-ray crystallography, involves aligning protein molecules into a lattice of repeating series of ‘unit cells’, and then passing x-rays through the resulting crystal. The structure of the protein is ‘solved’ by analyzing the resulting diffraction pattern.
|
|
But proteins are of irregular shape and the protein lattice is held together only by relatively weak electrostatic forces. So protein crystals are generally fragile and highly sensitive to environmental conditions. These must be adjusted to optimum levels for crystallization. At best it takes several hours to grow crystals suitable for data collection, but typically it takes months. Thus, protein crystallization has proved a major bottleneck in the whole-cell project.
|
|
In an attempt to increase efficiency, the researchers used 18 sample proteins from Thermus to test the capabilities of robots which use different techniques to crystallize proteins—sitting-drop vapor diffusion, hanging-drop vapor diffusion and a modified microbatch technique. They also trialed a microfluidic device designed to rapidly determine the best initial conditions, but which could not produce crystals in large enough quantities for diffraction.
|
|
The research team found that both vapor diffusion robots produced diffraction-quality crystals quicker than the microbatch robot—the sitting-drop being the faster. The microbatch robot, however, was most likely to be successful. The microfluidic device outperformed the other three on both counts. On the basis of these results the researchers used a combination of a sitting-drop and a microbatch robot to successfully determine structures for 360 of 944 purified proteins for the whole-cell project.
|

