| Posted: September 27, 2008 |
Bacterial nanoinjectors promise for drug delivery |
|
(Nanowerk News) Many pathogenic bacteria use a complex protein assembly - a bacterial nanoinjector resembling a syringe with a needle - to inject virulence proteins into their target cells to initiate infections. Among these pathogens is Salmonella typhimurium, which this summer caused the largest food-borne outbreak in US history by sickening more than 1400 people. US authorities suspected tainted peppers and tomatoes, and American farmers lost an estimated US $250 million in sales.
|
|
Other notable pathogens with nanoinjectors include Yersinia pestis, the cause of bubonic plague; Pseudomonas aeruginosa, which forms biofilms in lungs and is the leading cause of mortality among cystic fibrosis patients; Escherichia coli and Shigella species, responsible for many outbreaks of diarrhoeal diseases worldwide; and Burkholderia pseudomallei, a potential bioterrorism agent. No vaccines are currently approved for general use against any of these pathogens and, more alarmingly, many have developed multidrug resistance to current antibiotics. There is now a critical need to develop novel antibacterial therapies to counter the threat these bacteria pose to public health ("Structural dissection of the extracellular moieties of the type III secretion apparatus" – free access article).
|
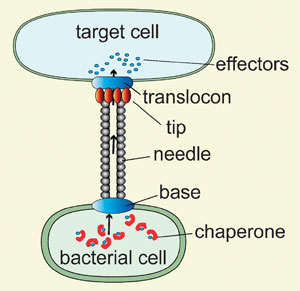 |
| The bacterial nanoinjector resembles a syringe with a needle and is used to inject virulence proteins into a target cell
|
|
Delivering proteins across cell membranes is a major challenge in drug development. Bacterial nanoinjectors transport proteins across three membranes: the inner and outer bacterial membranes plus the host cell membrane. Nanoinjectors essentially connect the bacterial cell contents with the host cell's and form a conduit for delivering bacterial effector proteins into the host. Once inside, these proteins reprogram the host's cellular functions to promote survival, growth, and bacterial propagation. (To prevent effectors from wreaking havoc inside the bacterium itself, chaperone proteins sequester them in a non-functional state.) Together, the nanoinjector, effectors and chaperones are known as the type III secretion system.
|
|
Nanoinjectors are assembled from more than 20 different proteins, and many show sequence and structural conservation across species. The base spans the two bacterial membranes and is assembled from about 9 different proteins; many are membrane proteins, and others show ring-like structures. From the base is anchored the needle, which is formed by the polymerisation of a small alpha-helical protein. The needle sits outside the main cell structure and measures about 50nm in length with a ~7nm outside diameter and a 2-3nm channel diameter. On top of the needle a tip complex serves as a platform for the translocon, which punctures a hole in the host cell membrane, allowing effector proteins to pass into the host.
|
|
In the past few years the atomic structures of several nanoinjector proteins have been determined by crystallography and NMR spectroscopy. Electron microscopy and crystallography-derived models of the base and the needle itself have also become available. Nevertheless, major challenges remain in understanding how nanoinjectors are assembled. During assembly, proteins have to pass through the narrow needle channel and deposit on top of the growing structure. How proteins are moved across the needle remains unknown. Another enigma is what powers this protein movement. Although there is an energy-releasing ATPase enzyme at the base, this is believed to unfold effectors from their chaperones to allow delivery. Nanoinjectors lack a motor that would propel proteins across.
|
|
Awareness of these structures presents us with opportunities. Researchers have used nanoinjectors to deliver proteins into cells. Also, because nanoinjectors are surface-exposed and critical to virulence, disrupting this apparatus is an attractive target for developing anti-infectives - drugs that will prevent infection but not kill bacteria. Anti-infectives potentially could solve problems associated with current antibiotics; namely antibiotic-resistance (because the kill rate is not 100 per cent, bacteria that survive have a selective advantage and propagate), and the indiscriminate killing of other bacteria, including those beneficial to the host. First discovered by microbiologists, the potential therapeutic uses of bacterial nanoinjectors mean they will continue to fascinate as they begin to catch the eyes of chemical biologists.
|

