| Posted: December 1, 2008 |
How plasma technology promises to greatly reduce the cost of fuel cell manufacture |
|
(Nanowerk News) During the past few years there has been much talk in the media about hydrogen-powered vehicles providing a green alternative to petrol engines. Some cities such as Perth, have even introduced trial hydrogen busses on their regular routes. But one of the current obstacles to this green revolution is the high cost of hydrogen fuel cell power plants.
|
|
A fuel cell is a device for converting fuel, usually in the form of a gas, directly into electricity. There are many possible forms of fuel cell, but proton exchange membrane (PEM) cells are widely seen as the most promising option for road vehicles. In a typical transport PEM cell, hydrogen and oxygen gas are fed to catalytic electrodes at opposite sides of a special membrane that is porous to protons but not electrons. The proton and electrons are separated by the action of a platinum catalyst in the electrodes. The protons can diffuse directly through the membrane but the electrons have to make their way through an external circuit to reach the other side, providing power for an electric motor in the process.
|
|
Current PEMs typically employ a membrane of Nafion, a sulfonated tetrafluorethylene copolymer developed by DuPont. The problem with both Nafion and platinum catalysts is that they're both very expensive to produce.
|
|
Professor Rod Boswell and the Space Plasma, Power & Propulsion Group at ANU have been working with plasmas for many years and recently became interested in the possibility using plasma deposition technology to dramatically reduce the cost of making fuel cells. "Production of current cells frequently relies on wet chemical stages which are messy, inefficient and consume large amounts of expensive materials. Our aim is to develop plasma based techniques to create both the membranes and the catalytic electrodes needed in fuel cells." Professor Boswell says.
|
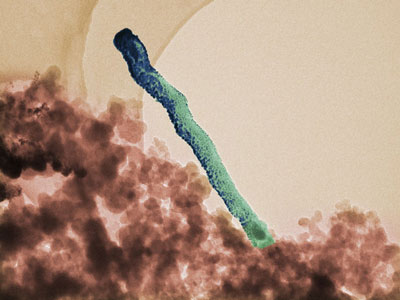 |
| False color transmission electron micrograph of platinum deposits on carbon nanostructures. The platinum is blue in this illustration.
|
|
The group has had a number of recent successes in production of both membranes and catalytic electrodes.
|
|
The manufacture of electrodes begins with a substrate of carbon paper; chosen because it's both porous to the gaseous fuels used in the final cells and is also an excellent conductor of electricity. This is loaded into the plasma reactor chamber and a very fine layer of nickel is deposited on the surface. Under the right conditions the nickel forms nanoscale droplets all over the carbon surface. The next stage is to introduce methane and hydrogen into the plasma chamber. Many complex reactions ensue leading to a very surprising situation where carbon complexes diffuse through the nickel seeds to form multi-carbon complexes below. The highly reactive hydrogen protons in the chamber etch away any carbon atoms that aren't strongly bonded to each other. The practical upshot of this is that carbon nano fibres grow below the nickel droplets lifting them from the substrate as they extend. The result is a carpet-like covering of carbon nanofibres on the paper.
|
|
Once the forest of nanofibres has been created the next step is to sputter coat the surface with platinum. "During the sputtering process the nanofibre tips get thickly coated with platinum with the droplets becoming progressively sparser further down the fibre. It's very much like snow falling in a forest, a lot gets deposited on the tree tops which greatly reduces the amount on the ground." Professor Boswell explains.
|
|
The tremendous advantage of this nanotechnology electrode is that its vast surface area and microscopically thin platinum coat reduce the amount of platinum required to about 15% of that in a conventional electrode of the same power specification.
|
|
The group has also succeeded in creating proton membranes by plasma decomposition of Trifluoromethanesulfonic (triflic) acid on a silicon wafer substrate. The finished membrane being detached from the wafer at the end of the process so it can be used for the next growth.
|
|
To make the finished fuel cell, the membrane is sandwiched between the hairy sides of two of the carbon catalytic electrode sheets and the whole assembly is hot pressed into a single sheet.
|
|
The new fuel cell technology is exciting stuff and may well be a key part of the transition to clean transport. But professor Boswell warns, "It has to be a holistic approach to clean transport. If you buy a cylinder of hydrogen today, chances are it was made from fossil fuels - it would be better to just burn the fossil fuel directly. What we need are fuel cell vehicles running on hydrogen that is inturn generated by clean electricity from solar or hydro. Then we'd be getting ahead."
|
|
At the moment it costs about six times as much to run on hydrogen as petrol. However, with petrol costs continuing to climb and the possibility of economies of scale in hydrogen production and distribution, it may not be all that long before that economic balance shifts.
|

