| Dec 04, 2012 |
Lipid metabolism regulates the activity of adult neural stem cells
|
|
(Nanowerk News) Neural stem cells generate thousands of new neurons every day in two regions of the adult brain: the subventricular zone of the lateral ventricles and the dentate gyrus of the hippocampus. This process, called adult neurogenesis, is critical for a number of processes implicated in certain forms of learning and memory. Impaired adult neurogenesis has been associated with a number of diseases such as depression, epilepsy, and Alzheimer’s disease.
|
|
A team led by Sebastian Jessberger, Professor of Neurosciences at the Brain Research Institute, has now identified a novel mechanism that plays a key role in adult neurogenesis and is required for the life-long activity of neural stem cells. Prof. Jessberger believes that “this finding will hopefully give us a new target to develop novel drugs against depression or neurodegenerative diseases”. The results of this study were published on December 2nd in the scientific journal Nature ("Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis").
|
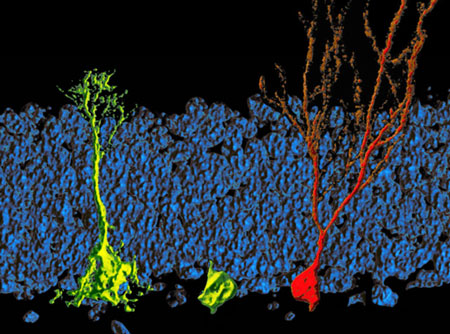 |
| A dormant stem cell (left, with extensions) is activated and starts cell division. The key for growth and development of the dividing cell (middle, no extensions) to the adult nerve cell (red, right), is a massive increase of fatty acid synthesis. (Image: Simon Braun, HiFo, UZH
|
|
Stem cells produce their own lipids
|
|
Researchers in his group could show that stem cells depend on glucose-derived production of new fatty acids and lipids. When the key enzyme of this pathway, fatty acid synthase (Fasn), is blocked in neural stem cells, they loose their ability to divide which results in a reduction in newborn neurons.
|
|
To prevent the constant division of neural stem cells, this pathway is regulated by a protein called Spot14, which inhibits lipid synthesis. Controlling Fasn activity is important to make sure that stem cells do not divide too often, which could lead to a premature exhaustion or depletion of the stem cell pool. Surprisingly, the metabolic state of neural stem cells seems to be fundamentally distinct from their daughter cells (newborn neurons) and other dividing cells in the central nervous system. These other cell types are able to take up lipids from the blood stream and use them as important structural components of cell membranes but also for signaling events and as an energy source.
|
|
Potential target for new drugs
|
|
The study published by the Jessberger group has identified a novel target to pharmacologically enhance the activity of neural stem cells in diseases that are associated with reduced levels of newborn neurons, such as depression.
|
|
Marlen Knobloch, postdoc in the Jessberger lab and first author of the study, says: “Currently, we have to understand in much greater detail why neural stem cells are in this distinct metabolic state; to this end, we are currently performing experiments in the lab with the aim to enhance neurogenesis through manipulation of lipid metabolism”. However, one must not place too high expectations for the quick development of novel drugs, although for Simon Braun, co-first author of the study, “the hope certainly is to increase the number of newborn neurons by targeting lipid metabolism in the human brain”.
|
|
Several institutions were involved in the current study. Besides the ETH Zurich and the University of Zurich, groups from the EPFL Lausanne and a number of scientists from abroad participated. Sebastian Jessberger was until this August an Assistant Professor at the Institute for Molecular Health Sciences at the ETH Zurich and now heads a laboratory at the Brain Research Institute of the University of Zurich.
|

